Method for determination of SARS virus nucleocapsid protein, reagent kit for the determination, test device, monoclonal antibody directed against SARS virus nucleocapsid protein, and hybridoma capable
A technology of SARS virus and nucleocapsid protein, applied in the field of hybridoma, can solve the problems of low sensitivity and insufficient sensitivity, and achieve the effect of sensitive detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0070] [V] Production of monoclonal antibody: The method for obtaining monoclonal antibody from hybridoma can be appropriately selected according to the required amount of monoclonal antibody and the properties of hybridoma. For example, there is a method of obtaining from the ascites in the peritoneal cavity of a mouse inoculated with the hybridoma cells, a method of obtaining from the culture supernatant by cell culture, and the like. Monoclonal antibodies at high concentrations of several mg / ml can be obtained from ascites fluid as long as they are hybridomas that can proliferate in the peritoneal cavity of mice. Hybridomas that cannot proliferate in vivo obtain monoclonal antibodies from the culture supernatant of cultured cells. The production of monoclonal antibodies obtained from cultured cells is lower than in vivo, but it has the advantages of less mixing of immunoglobulins and other impurities contained in the peritoneal cavity of mice, and it is easy to purify.
[...
Embodiment 1
[0076] Example 1: Production of Monoclonal Antibodies to SARS-NP
[0077] The monoclonal antibody of this example was produced through the following steps [I] to [V]. Specifically, [I] using genetic engineering technology to prepare antigen solution containing recombinant SARS-NP, [II] immunizing mice with this antigen solution, [III] fusing splenocytes and myeloma cells obtained from immunized mice, [IV] Select cells producing antibodies specific to SARS-NP from the obtained hybridoma cells, [V] proliferate the hybridomas in the peritoneal cavity of mice, and isolate monoclonal antibodies from the ascites. The details are as follows.
[0078] [I] Preparation of antigen solution
[0079] First, using the gene analysis software BioEdit version 7.0.0 (BioEdit Company), the base sequence of the nucleocapsid protein cDNA of the SARSTOR2 strain (published in the American Gene Database (accessionNumber; AY274119, protein id; AAP41047.1)) was expressed in Escherichia coli Convert ...
Embodiment 2
[0157] Embodiment 2: application in immunochromatography
[0158] Immunochromatography was performed using the nine kinds of monoclonal antibodies obtained in Example 1 (monoclonal antibody Nos. 1, 2, 3, 12, 13, 14, 15, 16, and 17).
[0159] (Production of test equipment for immunochromatography)
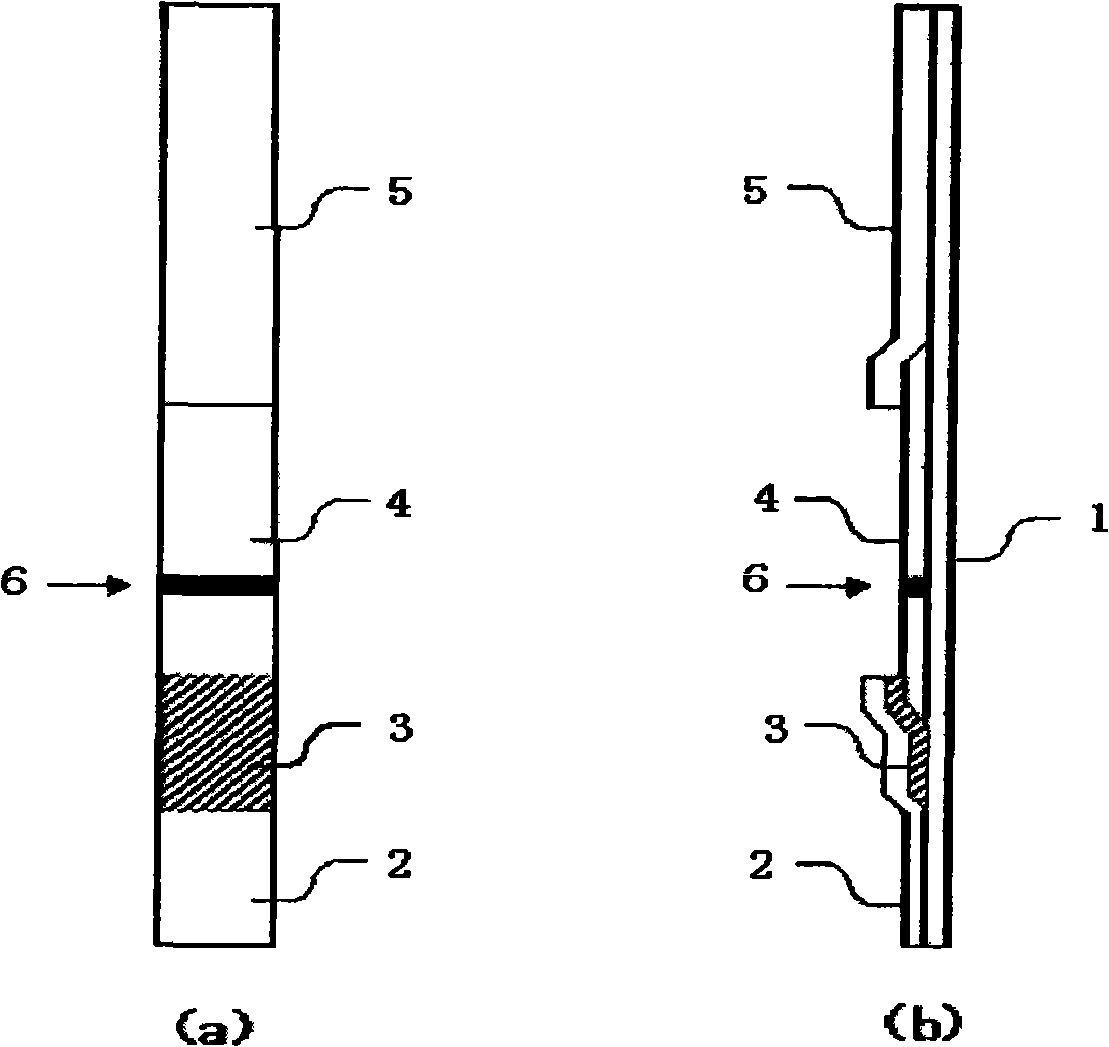
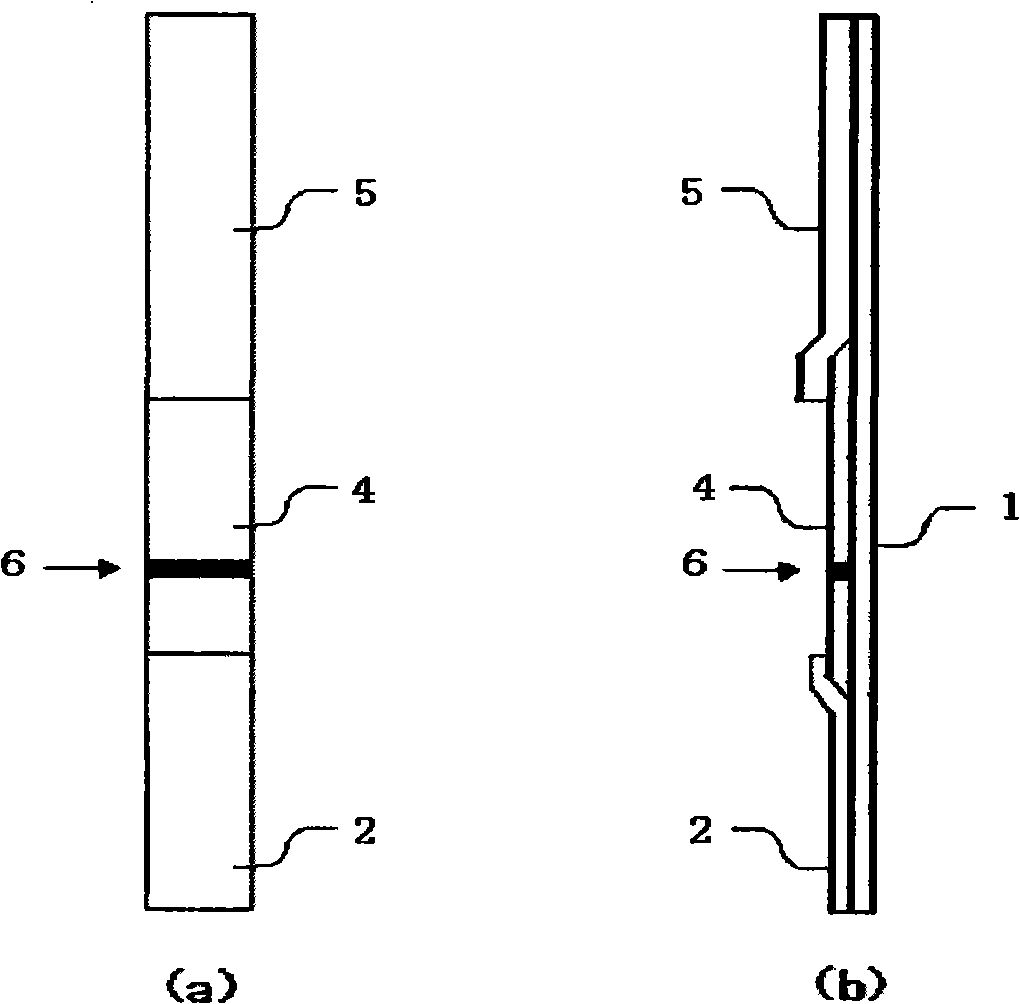
[0160] In this example, the figure 2 A test appliance of the shape shown. The substrate 1 of the test device of this example used a gasket with an adhesive surface, the absorbent layer 5 used Waterman paper WF1.5, and the carrier 4 for chromatography used a nitrocellulose membrane. The chromatography carrier 4 has a determination device 6 immobilized with any one of the above nine kinds of monoclonal antibodies. In this example, the sample application layer of the test equipment is immersed in the measurement sample prepared from the specimen, and the sample solution is diffused to the judgment device by capillary phenomenon.
[0161] (Antibody Sensitive Glue Emulsion)
[0162...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com