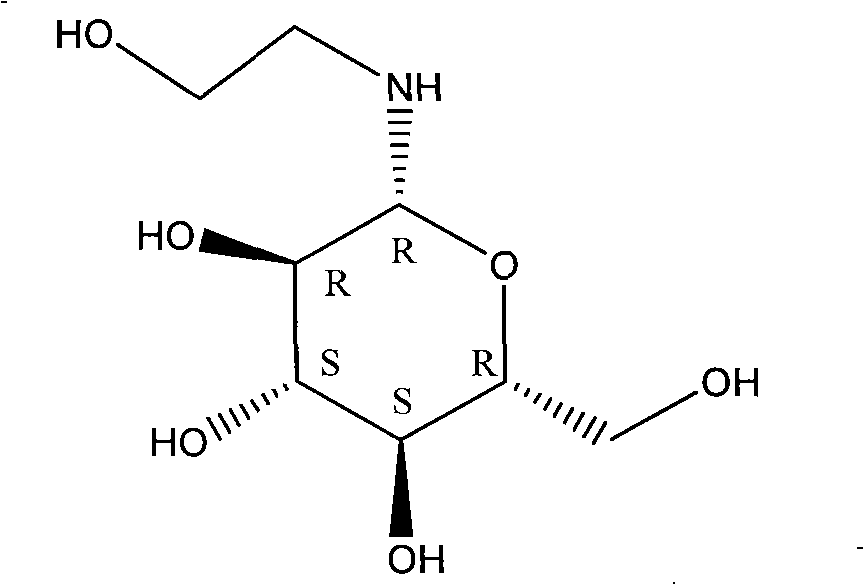
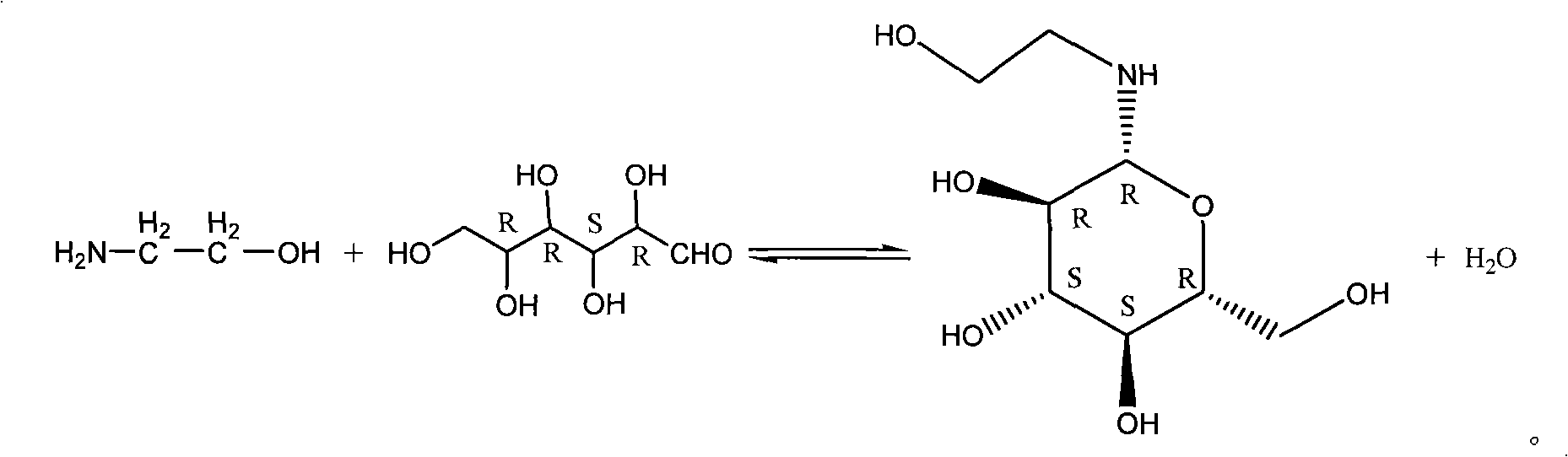
Piping production process of N-(2-hydroxyethyl)-beta-D-pyran glucosamine
A technology for glucopyranosamine and glucopyranose, which is applied in the field of preparing N--β-D-glucopyranosamine by using a pipeline reactor, can solve the problem of N-(2-hydroxyethyl)- The preparation process conditions and purification method of β-D-glucopyranosamine, the separation and purification method of N-(2-hydroxyethyl)-β-D-glucopyranosamine, and the strict requirements of process conditions are not involved, so as to achieve high purity , good color and high reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
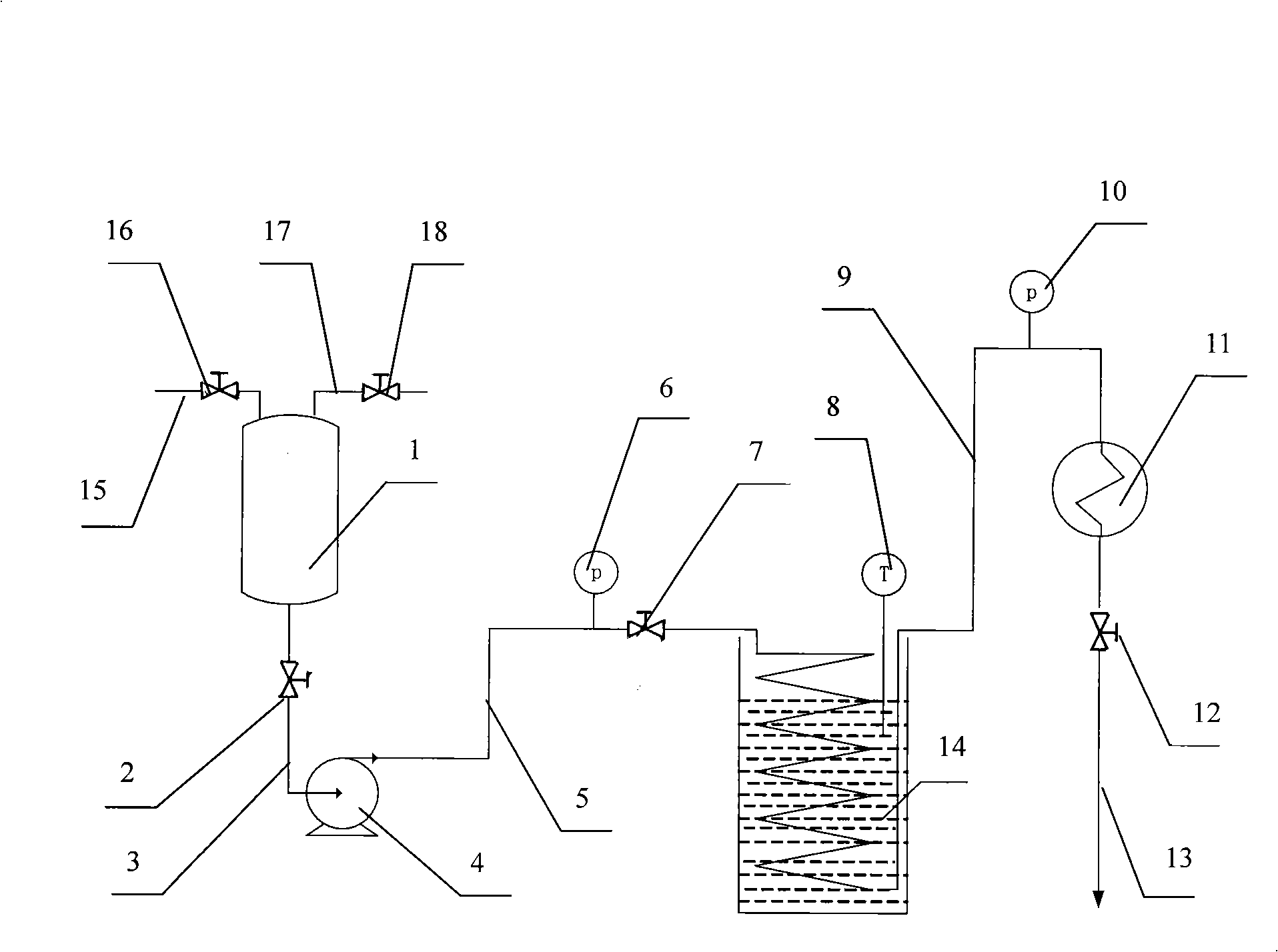
[0020] Embodiment 1, Fig. 1 have provided a kind of pipeline reactor, comprise raw material tank 1, metering pump 4, pipeline reactor 14 and condenser 11; The top of raw material tank 1 is provided with feed pipe 15 and discharge pipe 17 respectively , The feed pipe 15 is provided with a shut-off valve IV16, and the exhaust pipe 17 is provided with a shut-off valve V 18. The bottom outlet of the raw material tank 1 is connected with the metering pump 4 through the pipeline I3, and the metering pump 4 is connected with the inlet of the pipeline reactor 14 through the pipeline II5, and the outlet of the pipeline reactor 14 is connected with the inlet of the condenser 11 through the pipeline III9 Connected, the outlet of condenser 11 is connected with pipeline IV13. A cut-off valve I 2 is arranged on the pipeline I 3, a pressure gauge I 6 and a shut-off valve II 7 are respectively arranged on the pipeline II 5 according to the liquid flow direction, a thermometer 8 is arranged on...
Embodiment 2
[0023] Embodiment 2, a method for pipelined production of N-(2-hydroxyethyl)-β-D-glucopyranosesamine, adopts the pipeline reactor described in Example 1, and performs the following steps in sequence:
[0024] 1), raw material preparation:
[0025] Add glucose monohydrate 33g (0.17mol) in raw material tank 1, the solvent that each 50ml of methanol and water are formed; Shake well, glucose monohydrate is fully dissolved; Then add ethanolamine 13ml (0.20mol), shake well; Obtain mixing liquid.
[0026] 2), amination reaction:
[0027] Use the metering pump 4 to inject the above-mentioned mixed solution into the pipeline reactor 14, the reaction temperature is 60°C, and the reaction pressure is normal pressure; The reaction time is 140ml / h, that is, the reaction time is about 20mins. Follow up the reaction with TLC, the glucose point disappears, and the reaction ends, and the crude product solution containing N-(2-hydroxyethyl)-β-D-glucopyranosesamine.
[0028] 3), post-processi...
Embodiment 3
[0030] Embodiment 3, a method for pipelined production of N-(2-hydroxyethyl)-β-D-glucopyranosesamine, adopts the pipeline reactor described in Example 1, and performs the following steps in sequence:
[0031] 1), raw material preparation:
[0032] Add anhydrous glucose 30g (0.17mol) in raw material tank 1, the solvent that methanol 60ml and water 50ml form; Shake up, make anhydrous glucose fully dissolve; Then add ethanolamine 13ml (0.20mol), shake up; Obtain mixing liquid.
[0033] 2), amination reaction:
[0034] Use the metering pump 4 to inject the above-mentioned mixed solution into the pipeline reactor 14, the reaction temperature is 70°C, and the reaction pressure is 1.0Mpa; The reaction time is 180ml / h, that is, the reaction time is about 15mins, and the reaction is tracked by TLC. The glucose point disappears, and the reaction is ended, and the crude product solution containing N-(2-hydroxyethyl)-β-D-glucopyranosesamine is obtained.
[0035] 3), post-processing:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Inner diameter φ | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com