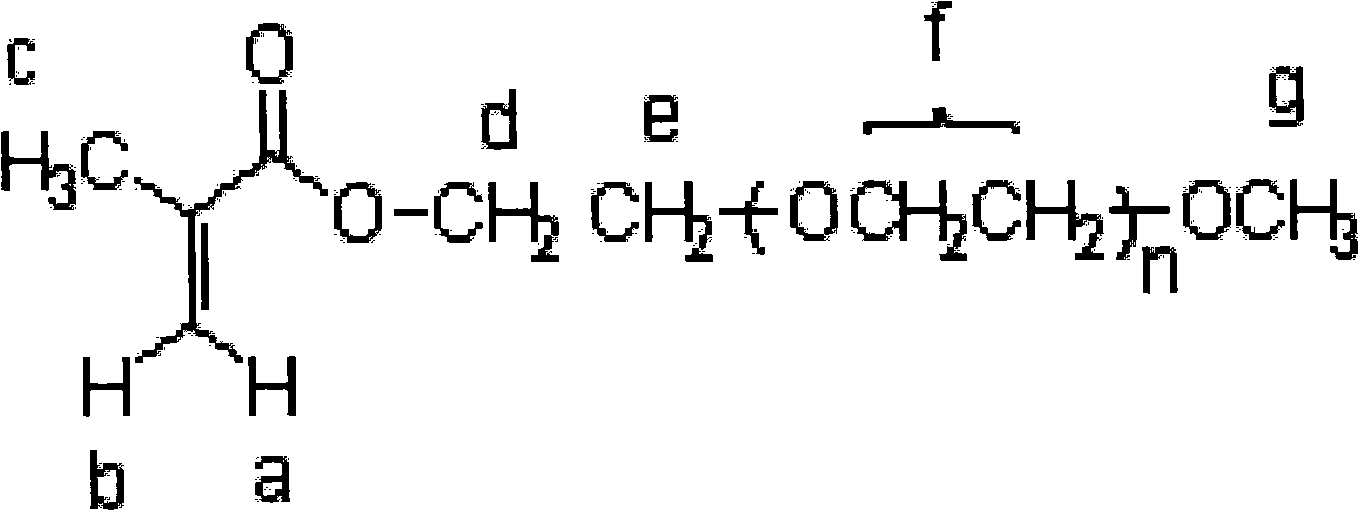Method for synthesizing polyethyleneglycol monomethyl ether metacrylic acid ester
A technology of polyethylene glycol monomethyl ether and methacrylate, which is applied in the field of synthesis of polyethylene glycol monomethyl ether methacrylate, can solve the problems of low esterification rate and Michael addition side reactions, which are difficult to avoid Polymerization and other problems, to achieve the effect of simple steps, increase the rate of esterification, avoid polymerization and other side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The experimental instruments are the instruments routinely used in the laboratory. Reagents are commercially available chemically pure or analytically pure chemical reagents.
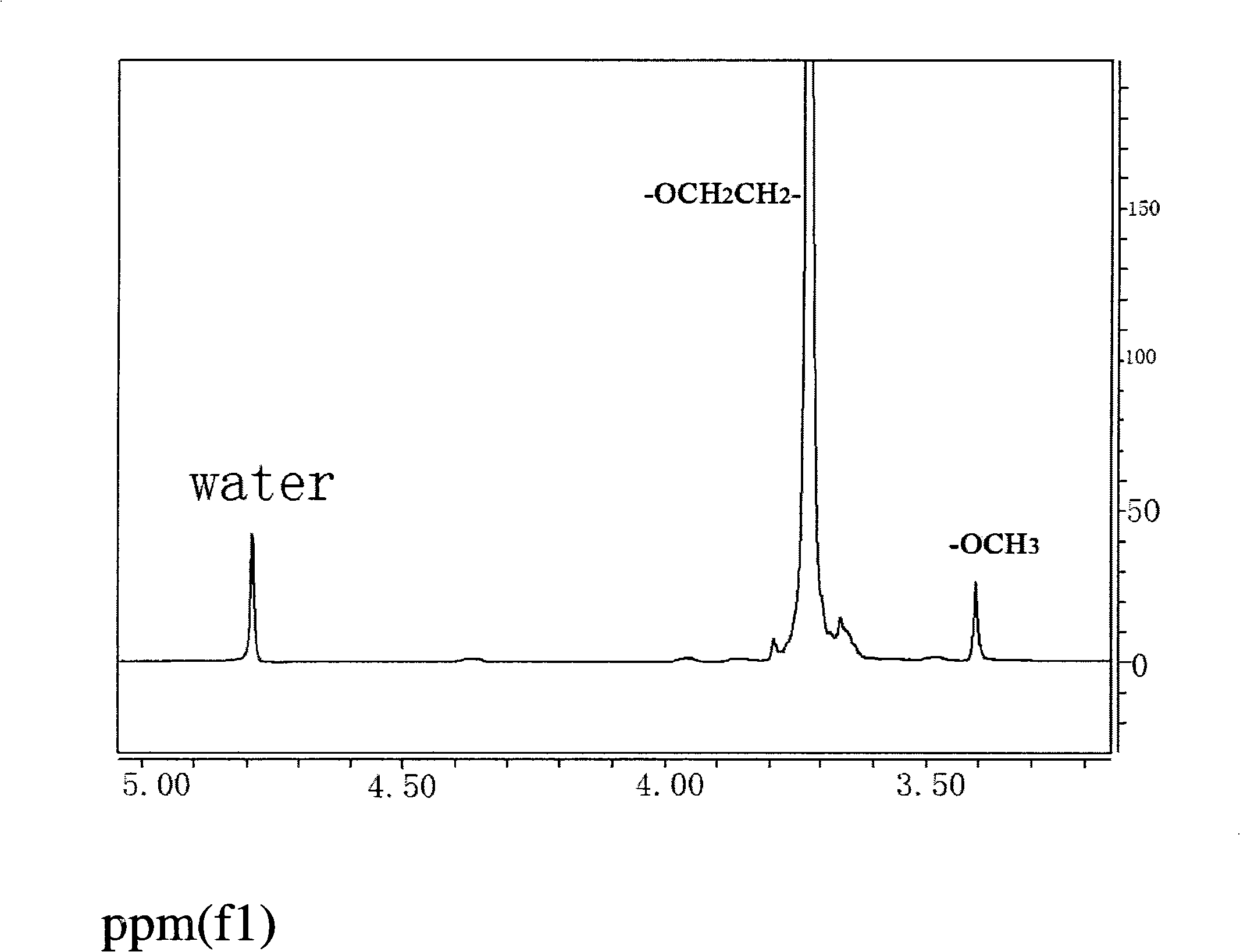
[0029] Raw material polyethylene glycol monomethyl ether (MPEG-400) 1 See attached for H NMR spectrum figure 1 , at δ~3.4ppm is the end of MPEG-OCH 3 proton peak, 3.7ppm for MPEG-OCH 2 CH 2 The proton peak of O-.
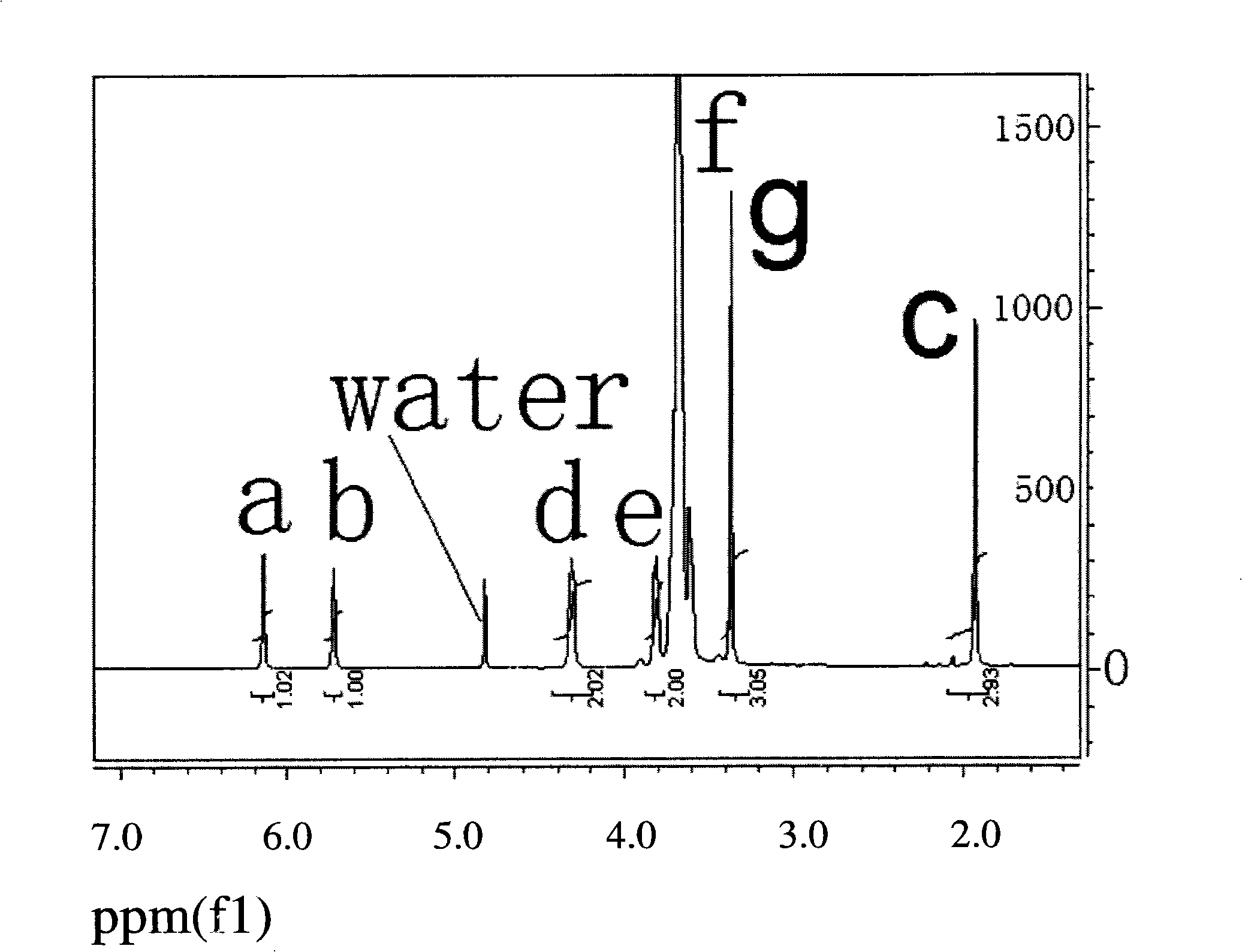
[0030] Using polyethylene glycol monomethyl ether and methyl methacrylate to synthesize the polyethylene glycol monomethyl ether methacrylic acid by transesterification under the condition of feeding air and in the presence of an alkali catalyst, a polymerization inhibitor and a reaction medium ester. Concrete preparation process is as follows:
[0031] (1) Add 60g polyethylene glycol monomethyl ether (M n =400, 0.15mol), 22.5g methyl methacrylate (0.225mol), 0.042g phenothiazine (0.2mmol), 0.036g lithium tert-butoxide (0.45mmol) and 40ml cyclohexane, start stirring, pass into ...
Embodiment 2
[0038] The experimental equipment and reagent requirements are the same as in Example 1. The specific synthesis process is as follows:
[0039] (1) Add 90g polyethylene glycol monomethyl ether (M n =600, 0.15mol), 30g methyl methacrylate (0.3mol), 0.042g phenothiazine (0.2mmol), 0.036g lithium tert-butoxide (0.45mmol) and 45ml cyclohexane, start stirring and feed air Raise the temperature and heat to reflux under certain conditions, and keep the total reflux for 10 minutes, then control the reflux ratio to 5:1~6:1;
[0040] (2) Add 0.018g of lithium tert-butoxide every 30 minutes to lower the temperature of the kettle to below 70°C, three times in total, add 0.021g of phenothiazine at the same time for the third time, and adjust the reflux ratio to 8:1 ~9:1.
[0041] (3) After 4 hours and 20 minutes of reactive distillation, cyclohexane and unreacted methyl methacrylate were distilled off under reduced pressure to obtain 104.1 g of a light yellow viscous liquid product.
...
Embodiment 3
[0045] The experimental equipment and reagent requirements are the same as in Example 1. The specific synthesis process is as follows:
[0046] (1) Add 65g polyethylene glycol monomethyl ether (M n =1000, 0.065mol), 30ml methyl methacrylate (0.28mol), 0.03g phenothiazine (0.15mmol), 0.028g lithium tert-butoxide (0.35mmol) and 45ml cyclohexane, start stirring and feed air (10-15mL / min) and heat up to reflux, keep the total reflux for 10min, and control the reflux ratio to 5:1~6:1;
[0047] (2) Add 0.014g lithium tert-butoxide after 1h, continue the reaction distillation for 1h, add 0.02g phenothiazine and 0.014g lithium tert-butoxide, and adjust the reflux ratio to 8:1~9:1;
[0048] (3) After co-reactive distillation for 3 hours and 15 minutes, cyclohexane and unreacted methyl methacrylate were distilled off under reduced pressure, and 71.2 g of light yellow semi-solid product was obtained by cooling.
[0049] During the reaction, add cyclohexane in due course to ensure that...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com