Process for preparing anti-hypertension polypeptides by enzymolysis of MRJP1 expression products of China honey bee
A technology of Chinese honeybee and expression products, applied in the fields of botanical equipment and methods, biochemical equipment and methods, animal/human peptides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
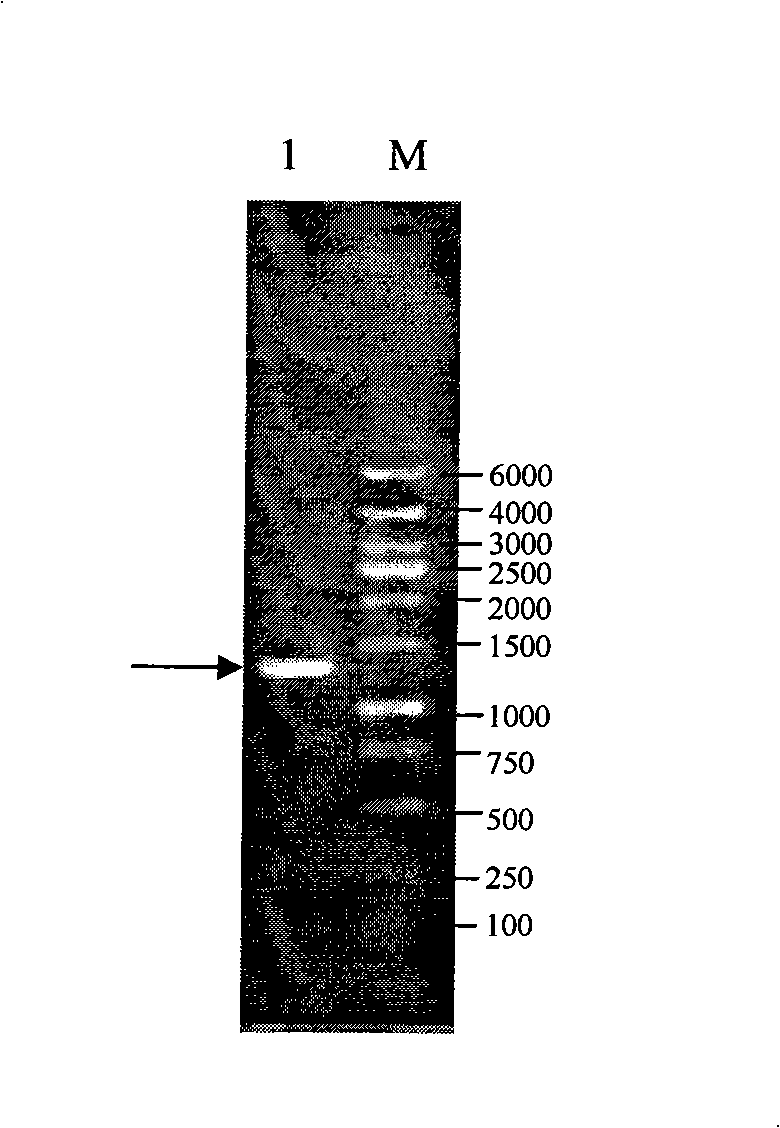
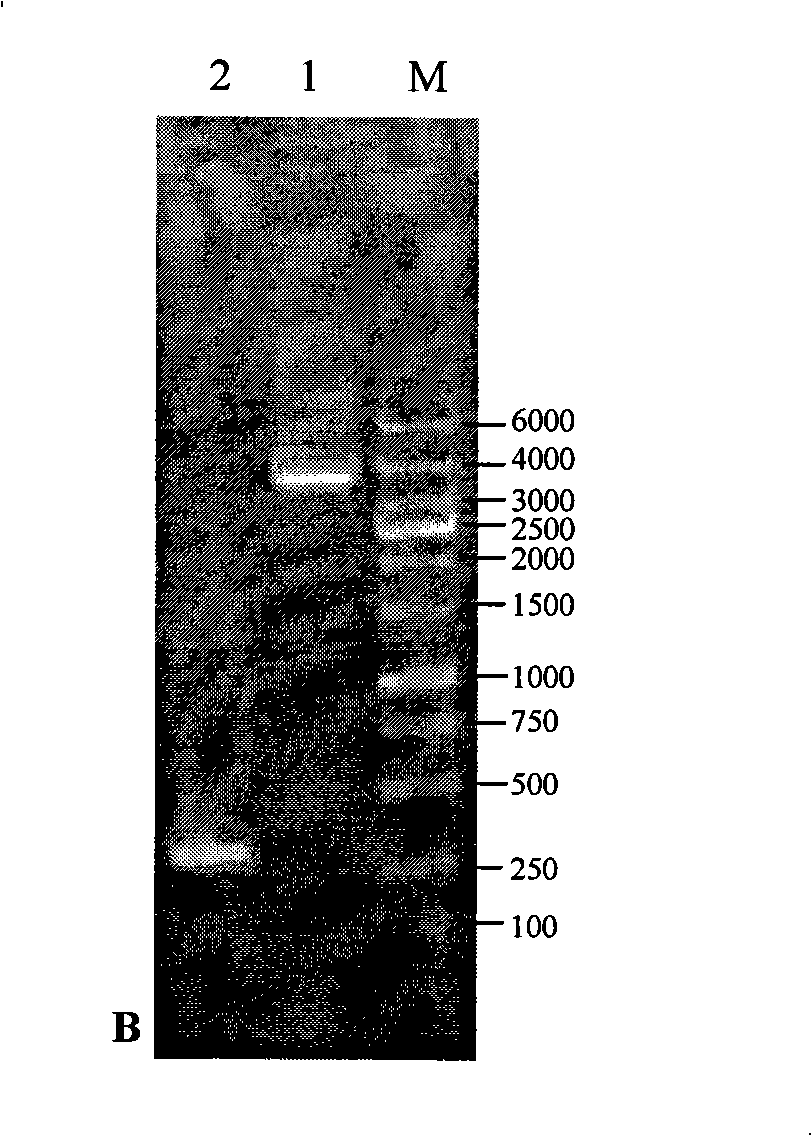
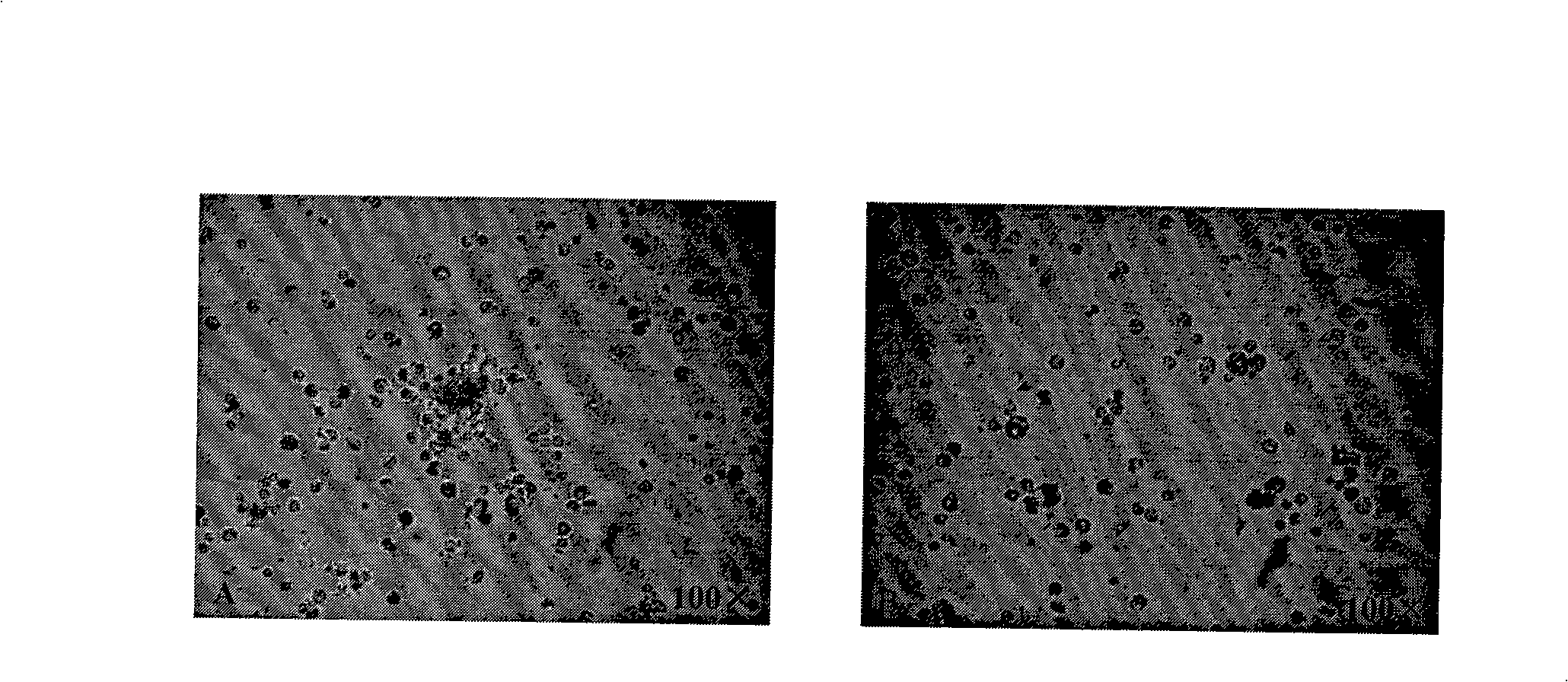
[0034] The preparation method of the eukaryotic expression product of Apis cerana MRJP1 that promotes cell growth is characterized in that the steps are as follows:
[0035] 1) Cloning of MRJP1 gene
[0036] a) Using the MRJP1 cDNA from the head of 8-day-old worker bees of Apis cerana as a template, using Primer Primer 5.0 software, the sequence of the upstream primer F-Prime was designed as 5'- CCATGG ACATGACAAGGTGGTTGTT-3', the downstream primer R-Prime sequence is 3'TAAAGTTATGTAGACATT CGCCGGCG -5', respectively introduce restriction sites Nco I and Not I into the upstream primer F-Prime sequence and the downstream primer R-Prime sequence (see the underlined part);
[0037] b) The PCR reaction system is 50 μl in total, and the composition is as follows: 5 μl 10×PCR Buffer, 6 μl 25mM MgCl 2 , 1μl 10mM dNTP Mix, 2μl 10μM F-Prime, 2μl 10μM R-Prime, 5μl Apis cerana MRJP1cDNA template, 0.75μl 5U / μl Taq DNA Polymerase, add ddH 2 O to a final volume of 50 μl;
[0038] The PCR ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com