Oxygen reduction catalyst for fuel cell and preparation method thereof
A fuel cell and catalyst technology, which is applied in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve the problems of increasing the manufacturing cost of oxygen reduction catalysts, hindering the commercial production of fuel cells, and complex synthesis processes. The effect of shortening the preparation time, strong controllability of the preparation process and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] At 0°C, add 40g of absolute ethanol and 0.0806g of cobalt chloride into a 100mL flask, add 0.2g of diethylenetriamine dropwise under stirring to coordinate to obtain cobalt diethylenetriamine chelate, add 0.1g of carbon black carrier Ketjen Black, continued to stir for 120 minutes, evaporated to remove the reaction medium; then under inert gas, heat treatment at 800 ° C for 90 minutes at a high temperature, and then cooled to obtain a carbon-supported cobalt diethylenetriamine as an oxygen reduction catalyst for fuel cells.
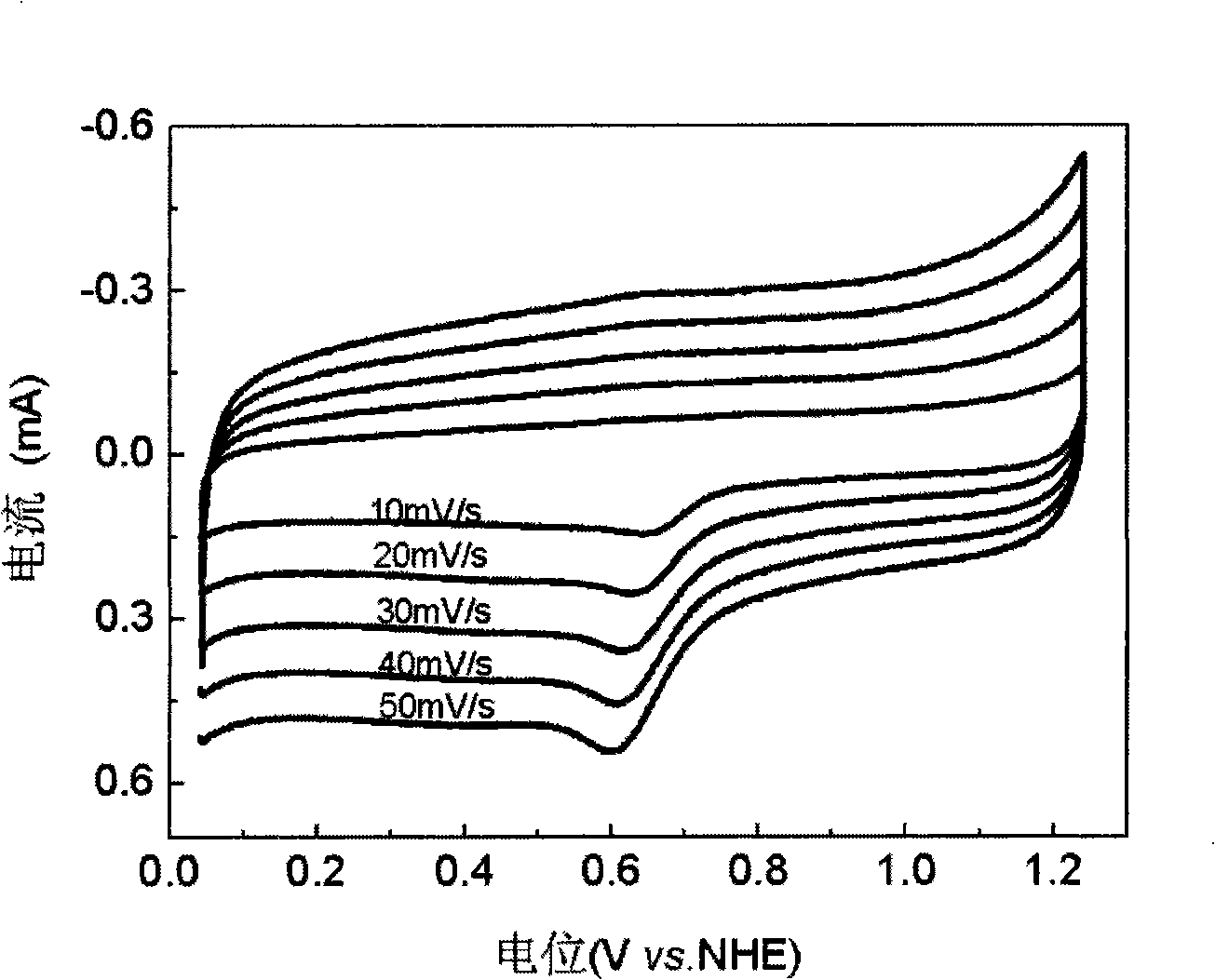
[0026] figure 1 It is the fuel cell oxygen reduction catalyst that embodiment 1 obtains in oxygen-saturated 0.5M H 2 SO 4 The electrochemical cyclic voltammetry test was carried out in the solution, and the scanning speed was 5mV / s, 10mV / s, 20mV / s, 30mV / s, 40mV / s and 50mV / s. It can be seen from the figure that with the increase of scanning speed, the peak potential of oxygen reduction shifts negatively, and the peak current increases obviously. ...
Embodiment 2
[0028] At 60°C, add 80g of absolute ethanol and 0.0844g of cobalt acetate to a 100mL flask, add 0.25g of diethylenetriamine dropwise under stirring to coordinate to obtain cobalt diethylenetriamine chelate, and add 0.1g of carbon black carrier Black Pearl 2000, continue to stir for 240 minutes, evaporate and remove the reaction medium; then under inert gas, heat treatment at 800°C for 60 minutes at high temperature, and then cool down to obtain a carbon-supported cobalt diethylenetriamine as an oxygen reduction catalyst for fuel cells.
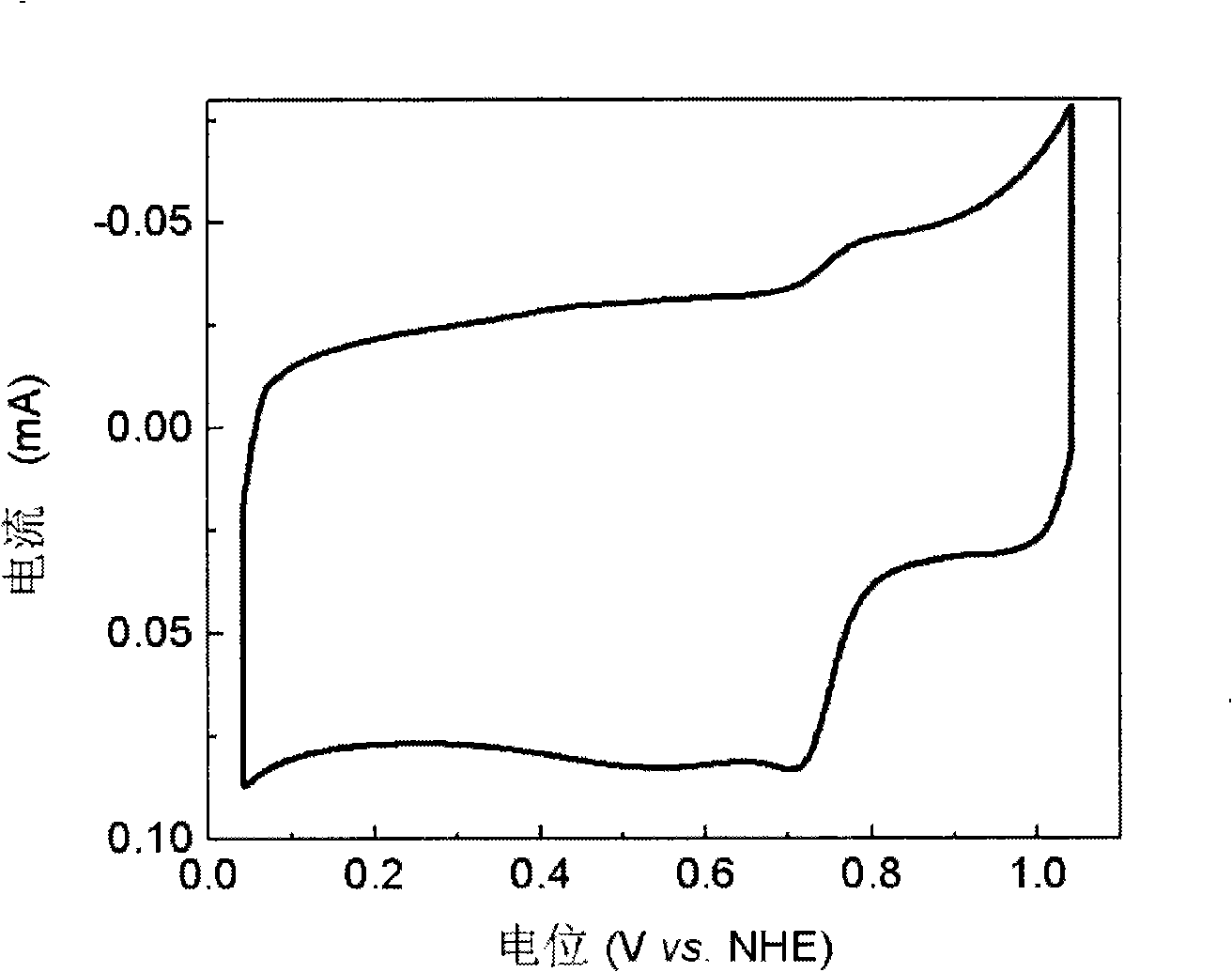
[0029] figure 2 It is the fuel cell oxygen reduction catalyst that embodiment 2 obtains in oxygen-saturated 0.5M H 2 SO 4 +0.5M CH 3 Cyclic voltammetry curve in OH solution, the scan speed is 5mV / s. The oxygen reduction peak potential reaches 0.714V (vs. NHE), and the oxygen reduction peak current reaches 0.083mA, indicating that the catalyst of the present invention has better oxygen reduction catalytic performance and methanol oxidation ...
Embodiment 3
[0031] At 25°C, add 40g of distilled water and 0.0924g of ferric chloride into a 100mL flask, add 0.25g of diethylenetriamine dropwise under stirring for coordination to obtain iron diethylenetriamine chelate, add 0.3g of carbon black carrier Vulcan XC -72R, continue to stir for 240 minutes, evaporate and remove the reaction medium; then under inert gas, heat treatment at 800°C for 90 minutes at high temperature, and then cool to obtain a carbon-supported iron diethylenetriamine as an oxygen reduction catalyst for fuel cells.
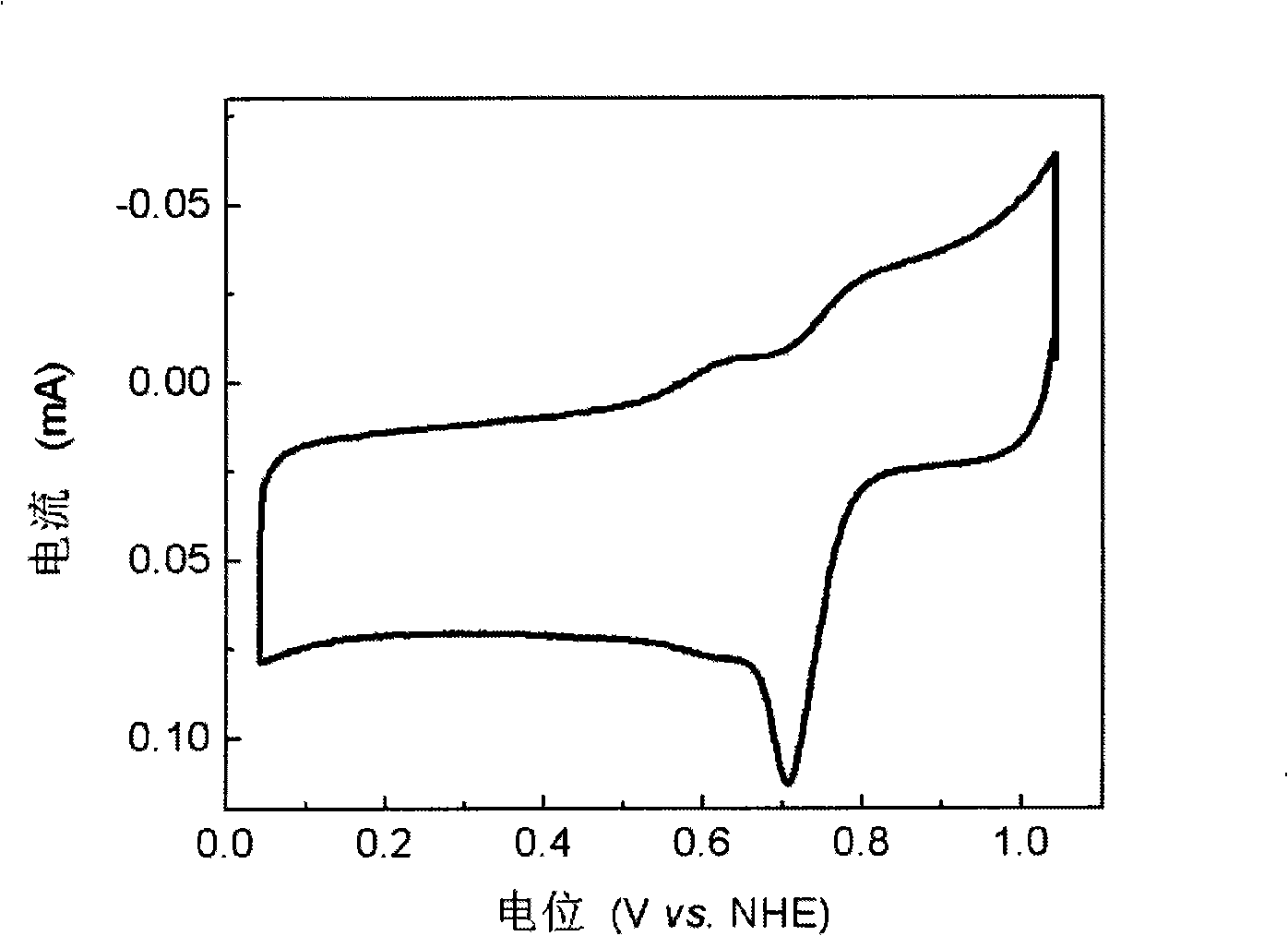
[0032] image 3 It is the fuel cell oxygen reduction catalyst that embodiment 3 obtains in oxygen-saturated 0.5M H 2 SO 4 Cyclic voltammetry curve in solution, the scan rate is 5mV / s. In the figure, there is an obvious characteristic peak of oxygen reduction, the peak potential is 0.706V (vs. NHE), and the peak current is 0.114mA, indicating that the catalyst of the present invention has better catalytic activity for oxygen reduction.
[0033] Figu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com