Particles with superparamagnetism and method for manufacturing same
A technology of superparamagnetic particles and manufacturing methods, applied in the direction of inorganic material magnetism, iron oxide/iron hydroxide, etc., can solve the problems of application limitations, inability to withstand high temperature and acid resistance for a long time, and achieve wide application and good acid resistance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Small-scale preparation of superparamagnetic iron oxide particles
[0062] Dissolve 0.25 grams of ferric chloride powder and 6 grams of urea crystal particles in 100 milliliters of deionized water, stir rapidly in a glass reactor equipped with a condenser tube for 10 minutes to form a yellow-green clear solution, and transfer it to an oil pan at 90°C for After 12 hours of reflux reaction, black iron oxide particles were formed. After standing for precipitation, the nearly clear supernatant was poured out, and the precipitate was washed three times with deionized water to remove ammonium ions and chloride ions left in the reaction. Prior to silica coating, the aforementioned precipitate was kept in an aqueous solution to avoid drying.
Embodiment 2
[0063] Example 2: Mass Preparation of Superparamagnetic Iron Oxide Particles
[0064]Dissolve 60 grams of ferric chloride powder and 90 grams of urea crystal particles in 1 liter (1000 ml) of deionized water, stir rapidly for 3 minutes in a glass reactor equipped with a condenser tube, and transfer the mixture to a temperature of 95°C The oil pan was refluxed for 12 hours to generate black iron oxide particles. After standing for precipitation, the nearly clear supernatant was sucked out, and the precipitate was washed three times with deionized water to remove the ammonium ions and chloride ions left in the reaction. Prior to silica coating, the aforementioned precipitate was kept in an aqueous solution to avoid drying.
Embodiment 3
[0065] Embodiment 3: coating of silicon dioxide
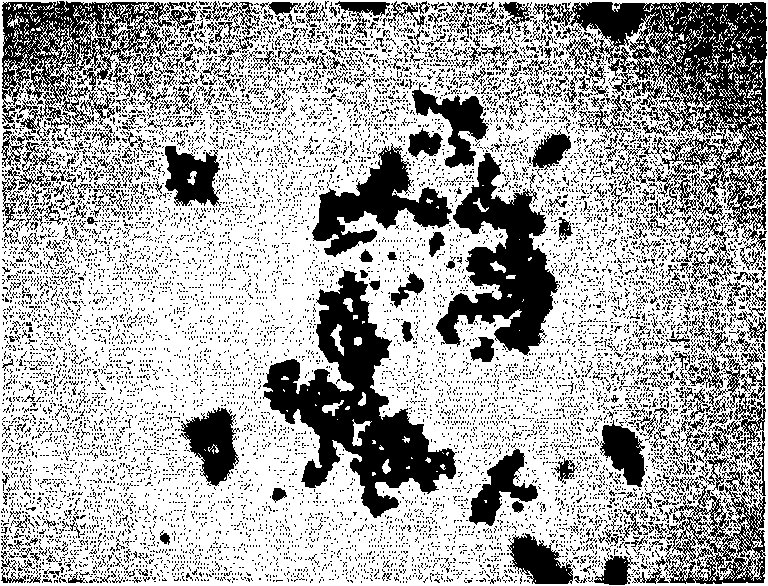
[0066] 30 grams of iron oxide particles prepared in Example 1 or Example 2 were dispersed in 225 milliliters of deionized water, then 22.5 milliliters of 28% ammonia water was added, and then 900 milliliters of isopropanol was added, and the solution was sealed in a glass reactor Stir continuously and ultrasonically shake for 10 minutes. Add 4.5 ml of tetraethyl silicate, raise the water temperature to 50°C, and continue the reaction for 2 hours; then lower the water temperature to room temperature (about 25°C), then add 4.5 ml of tetraethyl silicate, and continue the reaction 1 hour. After the reaction is completed, let it stand for precipitation, suck out the transparent supernatant, and wash it with deionized water until there is no smell of ammonia water and isopropanol. The image figure of the superparamagnetic particle coated with silicon dioxide that is made in embodiment 3 is as follows figure 1 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com