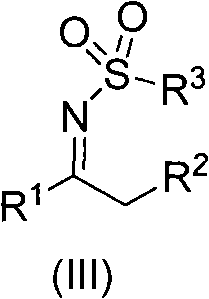
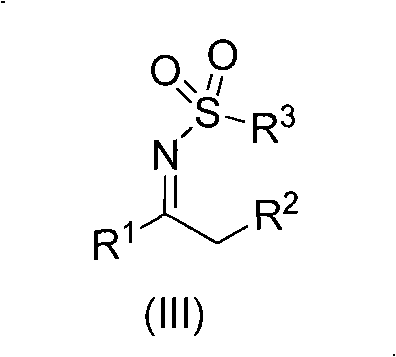
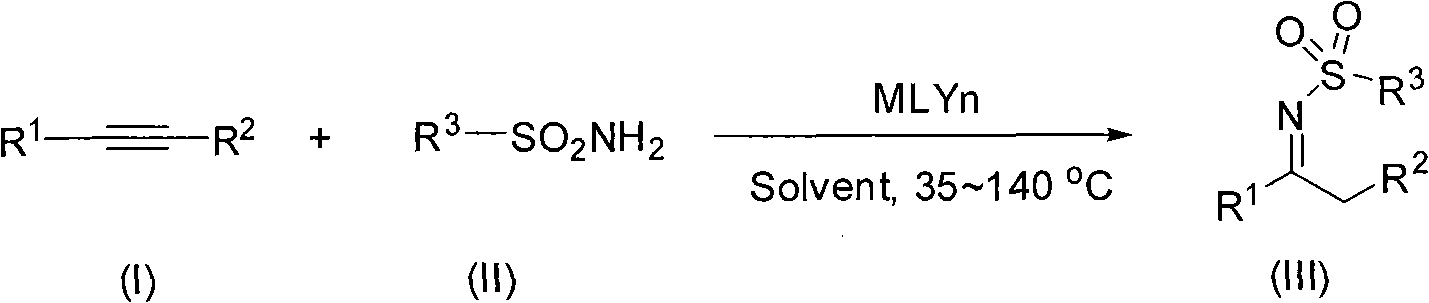N-sulfuryl ketimine compounds and preparation method thereof
A technology of sulfonyl imide and sulfonamide, which is applied in the preparation of sulfonic acid amide, chemical instruments and methods, organic compound/hydride/coordination complex catalyst, etc. products, limited applicability, etc., to achieve the effects of convenient operation, mild reaction conditions and good product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] N-(1-(4-ethylphenyl)ethylidene)benzenesulfonamide:
[0023]
[0024] P-ethylphenylacetylene (195.3mg, 1.5mmol), benzenesulfonamide (78.6mg, 0.5mmol), gold chloride complex ((PPh) 3 AuCl) (5 mg, 0.01 mmol), silver trifluoromethanesulfonate (10 mg, 0.04 mmol) were mixed in tetrahydrofuran (2.0 mL), heated in an oil bath at 100° C. for 6 h. Cool to room temperature, add saturated NaHCO 3 , and then extracted with ethyl acetate (50mL × 3), the organic layer was dried over anhydrous sodium sulfate, filtered, concentrated, and column chromatography (petroleum ether: ethyl acetate = 20: 1) gave 77 mg of the target compound with a yield of 53.6 %, yellow oily liquid.
[0025] 1 H NMR (500MHz, CDCl 3 ): δ8.06-8.03(m, 2H), 7.84(d, J=8.5Hz, 2H), 7.60-7.54(m, 3H), 7.23(d, J=8.5Hz, 2H), 2.98(s, 3H), 2.68(q, J=7.5Hz, 2H), 1.23(t, J=7.5Hz, 3H).
Embodiment 2
[0027] N-(1-(4-ethylphenyl)ethylidene)-4-iodobenzenesulfonamide:
[0028]
[0029] The operation was referred to Example 1, except that p-iodobenzenesulfonamide was used instead of benzenesulfonamide to obtain 130 mg of the target product as a yellow oily liquid with a yield of 63%.
[0030] 1 H NMR (500MHz, CDCl 3 ): δ7.90(d, J=8.0Hz, 2H), 7.83(d, J=8.0Hz, 2H), 7.75(d, J=8.0Hz, 3H), 7.24(d, J=8.0Hz, 2H ), 2.97(s, 3H), 2.69(q, J=7.5Hz, 2H), 1.23(t, J=7.5Hz, 3H).
Embodiment 3
[0032] 4-iodo-N-(1-(4-methoxyphenyl)ethylidene)benzenesulfonamide:
[0033]
[0034] The operation refers to Example 1, except that p-methoxyphenylacetylene is replaced by p-ethylphenylacetylene, p-iodobenzenesulfonamide is replaced by benzenesulfonamide, tetrahydrofuran is replaced by toluene, and 207 mg of the target product is obtained with a yield of 99.7%, a yellow oily liquid .
[0035] 1 H NMR (500MHz, CDCl 3 ): δ7.92-7.89(m, 4H), 7.75(d, J=8.5Hz, 2H), 6.90(d, J=9.0Hz, 2H), 3.87(s, 3H), 2.95(s, 3H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com