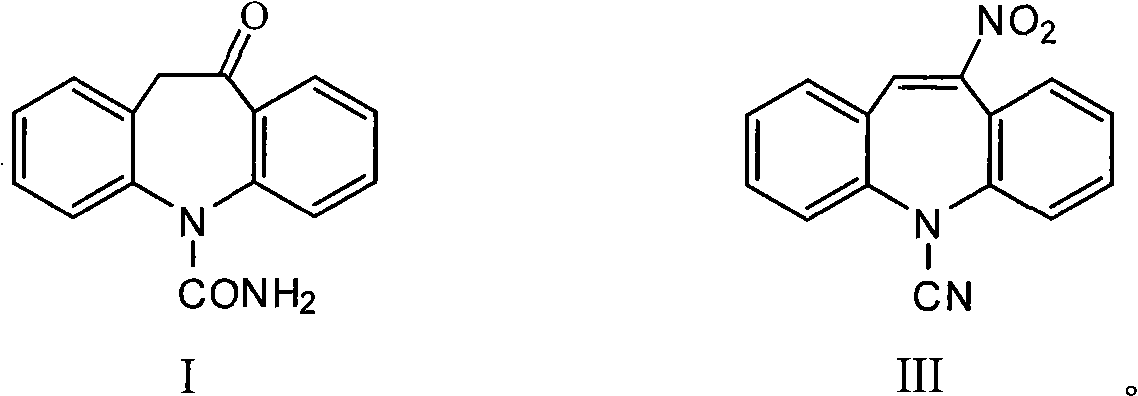
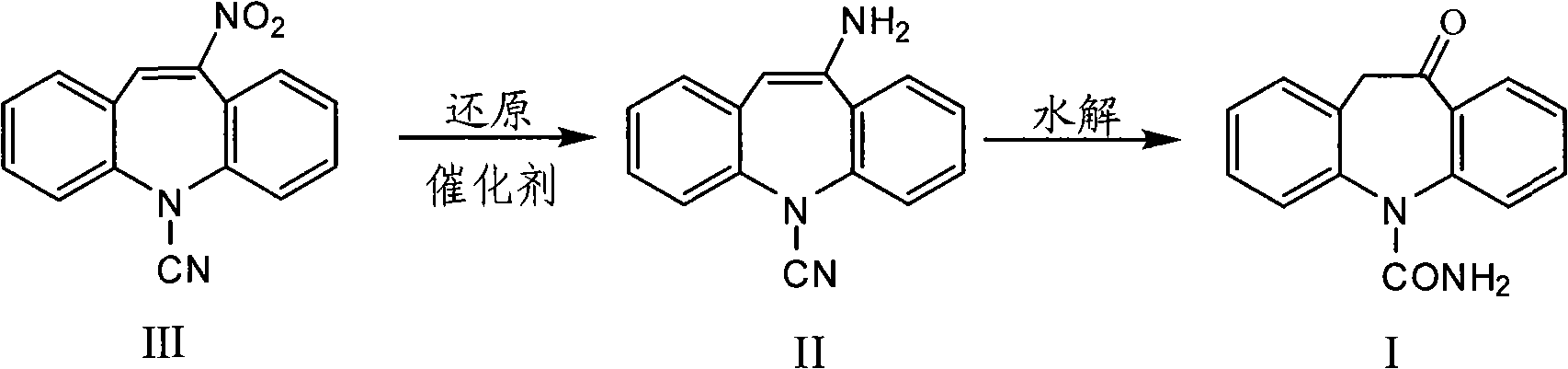
Chemical synthesis method for oxcarbazepine
A chemical synthesis and catalyst technology, which is applied in the field of chemical synthesis of oxcarbazepine, can solve the problems of low yield, unfavorable industrial production, environmental safety hazards and large cyanide, and achieve high reaction yield, no three wastes, and product purity Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Feeding amount 5-cyano-11-nitro-5H dibenzoazepine (hereinafter referred to as raw material III) 26.3g (0.1mol), the consumption of Raney nickel is 0.263g (0.01 times for the quality of raw material III), organic Solvent is toluene, and its consumption is 10 times of raw material III quality, and hydrogen pressure is 5 atmospheres.
[0028] Put the above materials into a 500mL autoclave reactor, replace the air three times, pass hydrogen to reach the specified pressure, raise the temperature to 40°C, and perform a reduction reaction for 5 hours. After the reaction is completed, filter and wash the filter residue with a solvent twice the mass of the raw material III , the filtrates were combined, the amount of concentrated hydrochloric acid was added dropwise to 8.3mL (0.1mol), and the reaction was kept for 5 hours, concentrated under reduced pressure until the solvent was basically evaporated, added 50mL of water, cooled to 5°C-10°C and allowed to stand for crystallizatio...
Embodiment 2
[0030] Feeding amount 5-cyano group-11-nitro-5H dibenzoazepine (III) 26.3g (0.1mol), the consumption of Raney nickel is 0.02 times of raw material III quality, and organic solvent is toluene, and its consumption is raw material 2 times of the quality of III, the hydrogen pressure is 10 atmospheres, and the mole of HCl in the concentrated hydrochloric acid is 0.8 times of the mole of raw material III.
[0031] The reduction reaction temperature was reflux temperature, the reduction reaction time was 4 hours, and the hydrolysis reaction time was 5 hours. Other operations were the same as in Example 1 to obtain 23.2 g of oxcarbazepine with a product yield of 92.0% and a purity of 99.1%.
Embodiment 3
[0033] Feeding amount 5-cyano group-11-nitro-5H dibenzoazepine (III) 26.3g (0.1mol), the consumption of Raney nickel is 0.10 times of raw material III quality, organic solvent is dichloromethane, its consumption It is 8 times of the mass of the raw material III, the hydrogen pressure is 15 atmospheres, and the mole number of HCl in the concentrated hydrochloric acid is 1.2 times of the mole number of the raw material III.
[0034] The reduction reaction temperature was reflux temperature, the reduction reaction time was 2 hours, and the hydrolysis reaction time was 5 hours. Other operations were the same as in Example 1 to obtain 21.6 g of oxcarbazepine with a product yield of 85.7% and a purity of 98.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com