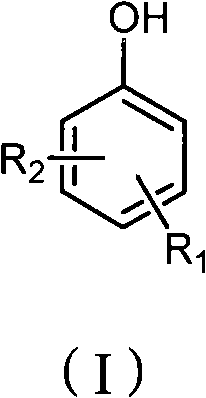
Method for preparing iodo-phenolic compounds
A phenolic compound and iodine technology is applied in the field of preparing iodinated phenolic compounds, can solve the problems of high price, complicated preparation of iodine donors and the like, and achieves the effects of low production cost, high use efficiency and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 2, the preparation of 6-dimethyl p-iodophenol
[0028] In a 250 ml glass reactor equipped with a stirrer, 2,6-dimethylphenol (10 g, 82 mmol) and 150 ml of dry methanol were added, potassium iodide (13.6 g, 82 mmol) and sodium hydroxide (3.3 g, 82 mmol) was stirred at 0°C for 10 minutes, then slowly added dropwise a solution of 30 ml of methanol solution in which trichloroisocyanuric acid (6.4 g, 28 mmol) was dissolved, and the addition was completed within 45 minutes, and the temperature was maintained at 0°C After 0.5 hours of reaction, the reaction solution was poured into 500 milliliters of cold water, filtered, and the filter cake was washed with dry methanol, and the filtrate was adjusted to pH=5 with dilute hydrochloric acid. At this time, a large amount of solids were precipitated, filtered, and the filter cake was washed with water. After drying, 18.4 g of 2,6-dimethyl-p-iodophenol was obtained after recrystallization from ethyl acetate and petroleu...
Embodiment 2
[0029] Embodiment 2 2, the preparation of 6-dichloro-p-iodophenol
[0030]In a 100 ml glass reactor equipped with a stirrer, add 2,6-dichlorophenol (1 g, 6.1 mmol) and 30 ml dry methanol, add potassium iodide (1.02 g, 6.1 mmol) and sodium hydroxide (0.24 g , 6.1 mmol), after stirring at 0°C for 10 minutes, slowly dropwise added 10 ml of methanol solution dissolved with trichloroisocyanuric acid (0.48 g, 2.06 mmol), and the dropwise addition was completed within 15 minutes, and the temperature was maintained at 0°C , after 0.5 hours of reaction, pour the reaction solution into 200 ml of water, filter, wash the filter cake with dry methanol, adjust the pH=4 of the filtrate with dilute hydrochloric acid, at this time, a large amount of solids are precipitated, filter, wash the filter cake with water, and dry , to obtain 1.44 g of 2,6-dichloro-p-iodophenol (yield 81%).
Embodiment 3
[0031] Example 3 Preparation of 3-methoxyl-4-hydroxyl-5-iodo-benzaldehyde
[0032] 100 mL glass reactor, equipped with a stirrer, add vanillin (1.4 g, 9.2 mmol) and 30 mL of dry methanol, add potassium iodide (1.53 g, 9.2 mmol) and potassium hydroxide (0.52 g, 9.2 mmol) , after stirring at 0°C for 10 minutes, slowly add 10 ml of methanol solution in which trichloroisocyanuric acid (0.71 g, 3.1 mmol) was dissolved, dropwise within 15 minutes, keep the temperature at 0°C, and finish the reaction in 2 hours Finally, the reaction solution was poured into 200 ml of water, filtered, and the filter cake was washed with dry methanol. The filtrate was adjusted to pH=3 with dilute hydrochloric acid, extracted with ethyl acetate, concentrated, and recrystallized with ethyl acetate and petroleum ether to obtain 2.4 g 3-methoxy-4-hydroxy-5-iodo-benzaldehyde (96% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com