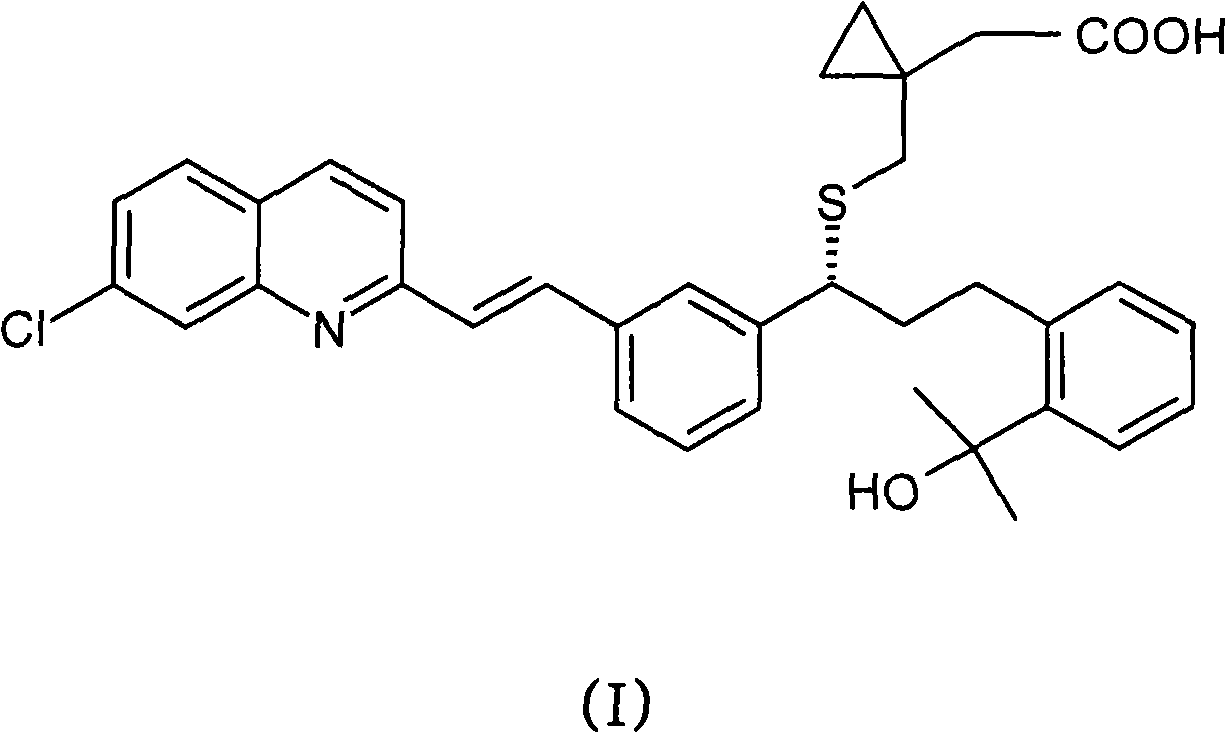
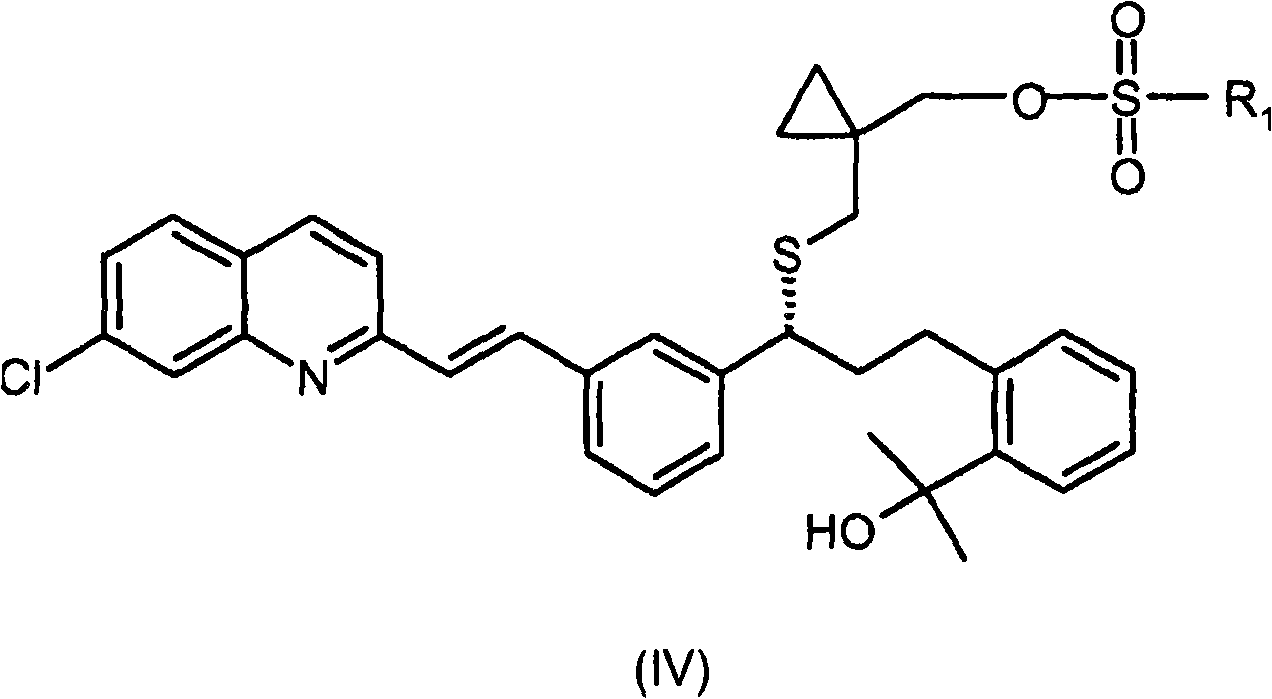
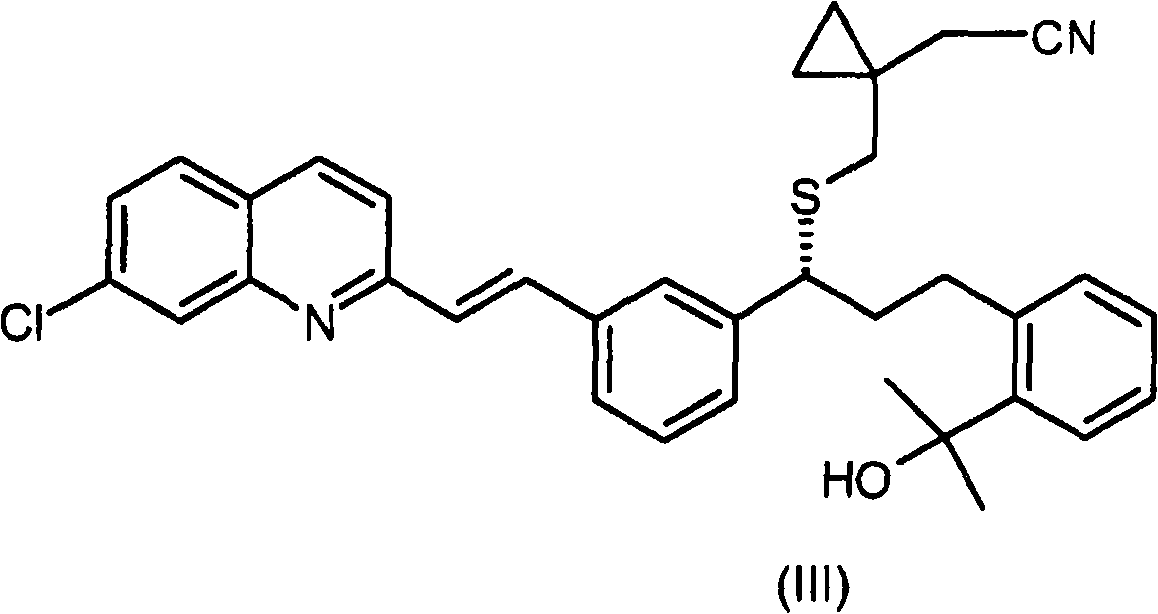
Process for the preparation of a leukotriene antagonist and intermediates thereof
A solvate, phase transfer catalyst technology, applied in the preparation of montelukast sodium salt, a new intermediate, the international non-proprietary name field of propane acetic acid, can solve the problem of lengthy chromatographic purification, not suitable for large-scale production, intermediate The problem of low yield of body and final product, etc., to achieve the effect of high chemical and optical purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The preparation of compounds of formula (VIII) involves the reaction of compounds of formula (XI) with potassium ethanethioate to give compounds of formula (X). Typically, this reaction is carried out using an excess of potassium thioacetate in a suitable solvent. Examples of suitable solvents include dimethylsulfoxide, dimethylformamide, ethyl acetate, acetonitrile or mixtures thereof. Preferably, the reaction is carried out at a temperature between room temperature and 70°C. After isolation of the compound of formula (X), it is hydrolyzed using an acid or base catalyst to give the compound of formula (VIII). Examples of suitable acids are hydrochloric acid, sulfuric acid, acetic acid and formic acid. Examples of suitable bases are hydroxides, carbonates and alkoxylates of alkali metals and alkaline earth metals. An example of a suitable solvent is (C 1 -C 6 )-alcohol, (C 6 -C 8 )-aromatic hydrocarbons, dimethylformamide, dimethylsulfoxide or mixtures thereof. ...
Embodiment 1
[0058] Embodiment 1: the preparation of S-(1-(hydroxymethyl) cyclopropyl) methyl thioacetate (X)
[0059] 200 g of 5,7-dioxa-6-thiaspiro[2.5]octane 6-oxide was dissolved in 1.2 liters of dimethylsulfoxide, and 308 g of potassium thioacetate was poured into the solution. The suspension was then heated at 40 °C for 5. Once the reaction was complete, a combination of 3.6 liters of ethyl acetate and 3.6 liters of water was added. The organic phase was separated, washed with water and concentrated to dryness in vacuo. 200 g of S-(1-(hydroxymethyl)cyclopropyl)methylthioacetate were recovered. Yield corrected by GC: 78%. 1 H NMR (400MHz, CDCl 3 ): 3.45 (2H, d, J: 6.4 Hz); 3.01 (2H, s); 2.53 (OH, broad triplet, J: 6.4 Hz); 2.39 (3H, s); 0.54 (4H, m).
Embodiment 2
[0060] Embodiment 2: the preparation of (1-(mercaptomethyl) cyclopropyl) methanol (VIII)
[0061] 200 g of S-(1-(hydroxymethyl)cyclopropyl)methylthioacetate were dissolved in 2 liters of methanol. Then 111 ml of concentrated HCl were added under nitrogen atmosphere and the solution was stirred at room temperature for 10. The solvent was distilled off and the residue was redissolved in 1.5 liters of dimethylformamide for the preparation of 2-((R)-3-(3-((E)-2-(7-chloroquinoline-2- yl)vinyl)phenyl)-3-(((1-(hydroxymethyl)cyclopropyl)methyl)thio)propyl)methyl benzoate. Yield: 100%. 1 H NMR (400MHz, CDCl 3 ): 3.57 (2H, s); 2.63 (2H, d, J: 8.0 Hz); 2.45 (OH, broad singlet); 1.43 (SH, t, J: 8.0 Hz); 0.52 (4H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com