Process for preparing cinnamate and derivates thereof
A technology of cinnamate and derivatives, which is applied in the field of preparation of cinnamate and derivatives thereof, can solve the problems of high preparation cost and difficult availability of acetate, and achieve the effects of low organic pollutants and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
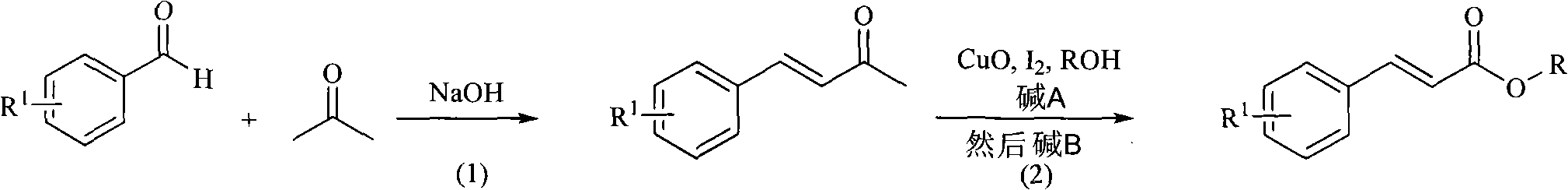
[0034] Example 1: preparation of
[0035] Dissolve 10.6g of benzaldehyde (0.1mol) in 50mL of acetone (acetone is in excess), then add 100mL of water, stir at 40°C, add dropwise 10mL of 5% NaOH solution, stir for 1.5 hours to stop the reaction, and use ethyl acetate Extract, dry with anhydrous sodium sulfate, and distill off the solvent under reduced pressure to obtain benzylidene acetone with a yield of 90.0%.
[0036] 6.4 g of copper oxide (0.08 mmol), 20.4 g of iodine (0.08 mmol), 13 mL of pyridine (0.16 mmol) and 100 mL of methanol (large excess of methanol) were added to 5.8 g of benzylidene acetone (0.04 mol) prepared above, Heated under reflux for 32 hours and then cooled, then added 11.0 g of potassium carbonate (0.08 mmol), and continued to reflux and stir for 12 hours to stop the reaction. After cooling, the insoluble matter was removed by filtration, and the filtrate was distilled to obtain the target product with a yield of 58.4%.
[0037] 1 H NMR (CDCl 3 , 60...
Embodiment 2
[0038] Example 2: preparation of
[0039] 6.4 g of copper oxide (0.08 mmol), 20.4 g of iodine (0.08 mmol), 13 mL of pyridine (0.16 mmol) and 100 mL of ethanol (large excess of ethanol) were added to 5.8 g of benzylidene acetone (0.04 mol) prepared above, Heated at reflux for 24 hours and then cooled, then added 11.0 g of potassium carbonate (0.08 mmol), and continued stirring at reflux for 12 hours to stop the reaction. After cooling, the insoluble matter was removed by filtration, and the filtrate was distilled to obtain the target product with a yield of 60.2%.
[0040] 1 H NMR (CDCl 3 , 600MHz), δ(ppm) 7.69(d, J=16.4Hz, 1H), 7.53-7.52(m, 2H), 7.39-7.37(m, 3H), 6.44(d, J=16.4Hz, 1H), 4.27(q, 2H), 1.34(t, 3H).
Embodiment 3
[0041] Example 3: preparation of
[0042] Add 6.4 g of copper oxide (0.08 mmol), 20.4 g of iodine (0.08 mmol), 13 mL of pyridine (0.16 mmol) and 100 mL of isopropanol (isopropyl Large excess of alcohol), reflux heating for 48 hours and then cooling, then added 11.0g of potassium carbonate (0.08mmol), continued reflux and stirring for 12 hours to stop the reaction. After cooling, the insoluble matter was removed by filtration, and the filtrate was distilled to obtain the target product with a yield of 62.8%.
[0043] 1 H NMR (CDCl 3 , 600MHz), δ(ppm) 7.67(d, J=16.4Hz, 1H), 7.53-7.51(m, 2H), 7.39-7.37(m, 3H), 6.42(d, J=16.4Hz, 1H), 5.14 (m, 1H), 1.30 (d, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com