Freeze-dried attenuated live vaccine for hepatitis A and its preparing process
A technology of live attenuated vaccines and hepatitis A, applied in antiviral agents, pharmaceutical formulas, medical preparations with non-active ingredients, etc., can solve problems such as low virus titer and large loss of vaccine activity, and achieve clear formula components , good safety and immunogenicity, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The lyoprotectant consists of the following ingredients: 2 grams of trehalose, 3.5 grams of glycine, dissolved in pure water to 100 ml;
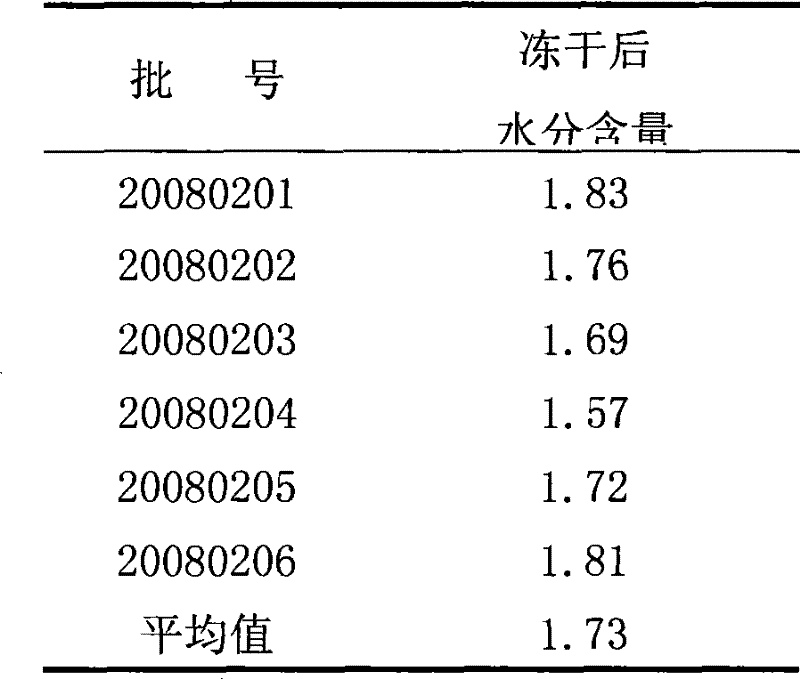
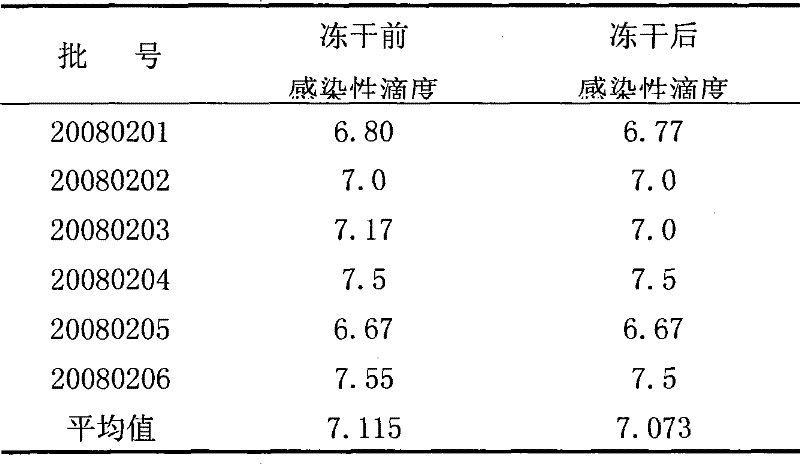
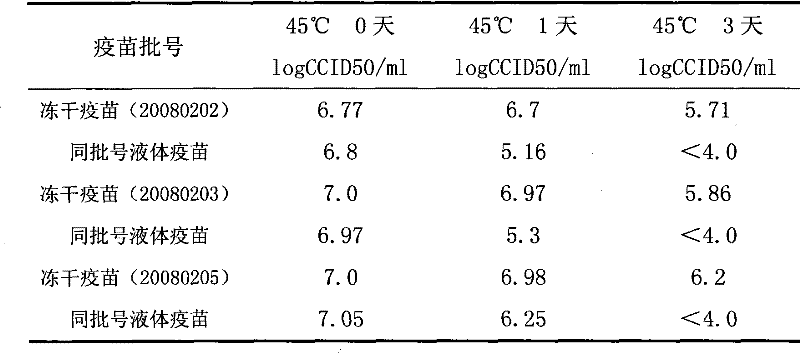
[0042] According to the volume ratio of hepatitis A live attenuated vaccine: lyoprotectant = 1:0.5, add the above-mentioned lyoprotectant to the hepatitis A live attenuated vaccine, mix and pack in vials, 0.5ml per bottle , put it into the chamber of the lyophilizer after half stoppering, lower the temperature to -40°C at a rate of 0.4°C / min, and maintain it for 4 hours; then raise the temperature to -15°C at a rate of 0.8°C / min; Vacuumize for 8 hours to maintain the temperature of the partition at -15°C; then raise the temperature to 25°C at a rate of 1.4°C / min, maintain for 8 hours, and finally press the plug and take it out of the box to obtain the freeze-dried live attenuated hepatitis A vaccine.
Embodiment 2
[0044] The lyoprotectant consists of the following ingredients: 5 grams of trehalose, 9 grams of glycine, dissolved in pure water to 100 ml;
[0045] According to the volume ratio of hepatitis A live attenuated vaccine: lyoprotectant = 1:1, add the above-mentioned lyoprotectant to the hepatitis A live attenuated vaccine, mix and pack in vials, 0.5ml per bottle , put it into the chamber of the lyophilizer after half stoppering, lower the temperature to -40°C at a rate of 0.5°C / min, and maintain it for 6 hours; then raise the temperature to -15°C at a rate of 1°C / min; Vacuum the vacuum for 10 hours, and maintain the temperature of the partition at -15°C; then raise the temperature to 25°C at a rate of 1.5°C / min, and maintain it for 8 hours; finally press the plug and take it out of the box to obtain the freeze-dried live attenuated hepatitis A vaccine.
Embodiment 3
[0047] The lyoprotectant consists of the following ingredients: 10 grams of trehalose, 16 grams of glycine, dissolved in pure water to 100 ml;
[0048] According to the volume ratio of hepatitis A live attenuated vaccine: lyoprotectant = 1:1, add the above-mentioned lyoprotectant to the hepatitis A live attenuated vaccine, mix and pack in vials, 0.5ml per bottle , put it into the chamber of the lyophilizer after half stoppering, lower the temperature to -40°C at a rate of 0.5°C / min, and maintain it for 6 hours; then raise the temperature to -15°C at a rate of 1°C / min; Vacuum the vacuum for 10 hours, and maintain the temperature of the partition at -15°C; then raise the temperature to 25°C at a rate of 1.5°C / min, and maintain it for 8 hours; finally press the plug and take it out of the box to obtain the freeze-dried live attenuated hepatitis A vaccine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com