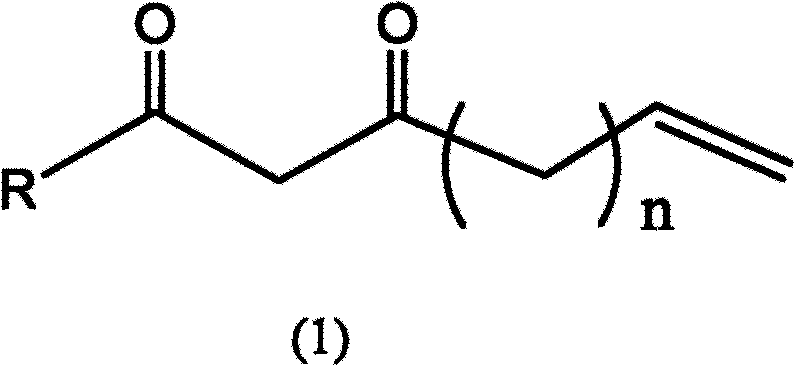
Beta-dione compounds with terminal vinyl group
A technology of compound and diketone, which is applied in the field of β-diketone compound, can solve the problems that there is no polymerization active center and cannot be used to prepare polymer light-emitting materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
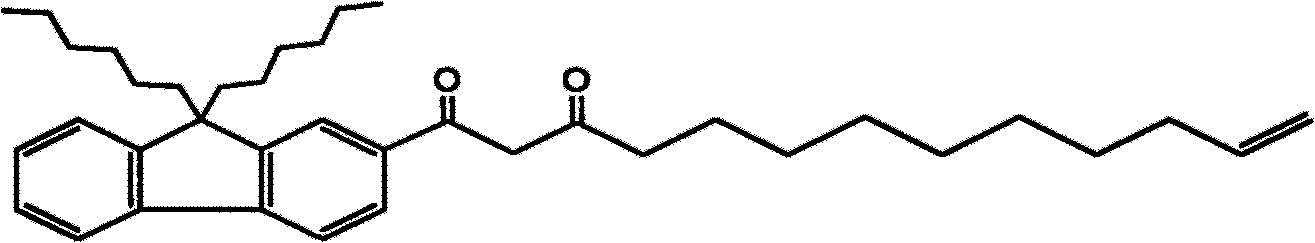
[0034] Example 1: Synthesis of 1-(9,9-dihexyl-2-fluorenyl)-12-tridecene-1,3-dione
[0035] 1.1 Synthetic route: the present invention is implemented with reference to the following synthetic route
[0036]
[0037]
[0038] 1.2 Synthesis steps
[0039] (1) Synthesis of 9,9-dihexylfluorene
[0040] Under nitrogen protection, add 10.00g fluorene and 200mL dimethyl sulfoxide to a 500mL three-necked flask, stir to dissolve, then add 25.3mL of 50% sodium hydroxide solution and 1.58g of tetrabutylammonium bromide. After stirring at room temperature for five minutes, 27.90 g of n-bromohexane was added dropwise. After the dropwise addition, the mixture was heated to 40° C. for 5 hours, and then naturally cooled to room temperature. Pour into 200mL of dichloromethane, stir and let stand, separate the organic phase and wash with water until neutral, dry with anhydrous magnesium sulfate, filter, concentrate the mother liquor, use petroleum ether as eluent, and separate by silica ...
Embodiment 2
[0056] Example 2: Synthesis of 1-(9,9-dihexyl-2-fluorenyl-7-octene-1,3-dione
[0057] 2.1 Synthesis
[0058] The operating steps of this example are basically the same as those in Example 1, except that hexyl hexenoate is used to replace hexyl undecylenate in the Claisen condensation reaction.
[0059] 2.2 Structure identification
[0060] Infrared spectrum (KBr, cm -1 ): 3070 (C=C-H); 1710 (C=O); 1590, 1470, 1449, 740 (fluorene ring); 2916, 2843, 1375 (-CH-).
[0061]NMR spectrum: δ(ppm), 16.20(s, 1H); 7.79-7.84(m, 2H); 7.55-7.59(m, 2H); 7.28-7.42(m, 3H); 6.70(s, 1H); 5.70 -5.78(m, 1H); 5.03-5.11(m, 1H); 4.93-4.98(m, 1H); 2.90-2.96(m, 2H); 1.93-1.98(m, 2H); 4H); 1.44-1.51 (m, 2H); 1.33-1.39 (m, 4H); 1.19-1.30 (m, 12H); 0.90-0.98 (m, 6H).
[0062] Elemental Analysis: C 33 h 44 o 2
[0063] Calculated value (%): C83.85, H9.38, O6.77;
[0064] Measured value (%): C83.78, H9.29, O6.71.
[0065] Mass Spectrum: 472(M + ).
[0066] The above data confirm that the compou...
Embodiment 3
[0069] Example 3: Synthesis of 1-(9,9-dihexyl-2-fluorenyl)-10-undecene-1,3-dione
[0070] 3.1 Synthesis
[0071] The operating steps of this example are basically the same as those in Example 1, except that hexyl nonenoate is used to replace hexyl undecylenate in the Claisen condensation reaction.
[0072] 3.2 Structural identification
[0073] Infrared spectrum (KBr, cm -1 ): 3075 (C=C-H); 1708 (C=O); 1598, 1476, 1450, 748 (fluorene ring); 2923, 2845, 1370 (-CH-).
[0074] NMR spectrum: δ(ppm), 16.25(s, 1H); 7.80-7.84(m, 2H); 7.53-7.58(m, 2H); 7.20-7.28(m, 3H); 6.70(s, 1H); 5.70 -5.75(m, 1H); 5.03-5.08(m, 1H); 4.91-4.97(m, 1H); 2.98(m, 2H); 1.89-1.96(m, 2H); 1.80-1.85(m, 4H) ; 1.37-1.40 (m, 2H); 1.28-1.33 (m, 6H); 1.21-1.25 (m, 16H); 0.91-0.96 (m, 6H).
[0075] Elemental Analysis: C 36 h 50 o 2
[0076] Calculated value (%): C83.99, H9.79, O6.22;
[0077] Measured value (%): C83.95, H9.71, O6.31.
[0078] Mass Spectrum: 514(M + ).
[0079] The above data confirm ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com