Medicament composition containing tirofiban and antithrombotic medicament active component
A technology for tirofiban and active ingredients, applied in the field of pharmaceutical compositions, can solve problems such as difficulty in oral administration, and achieve the effects of solving difficulty in oral administration, improving medication safety, and improving compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
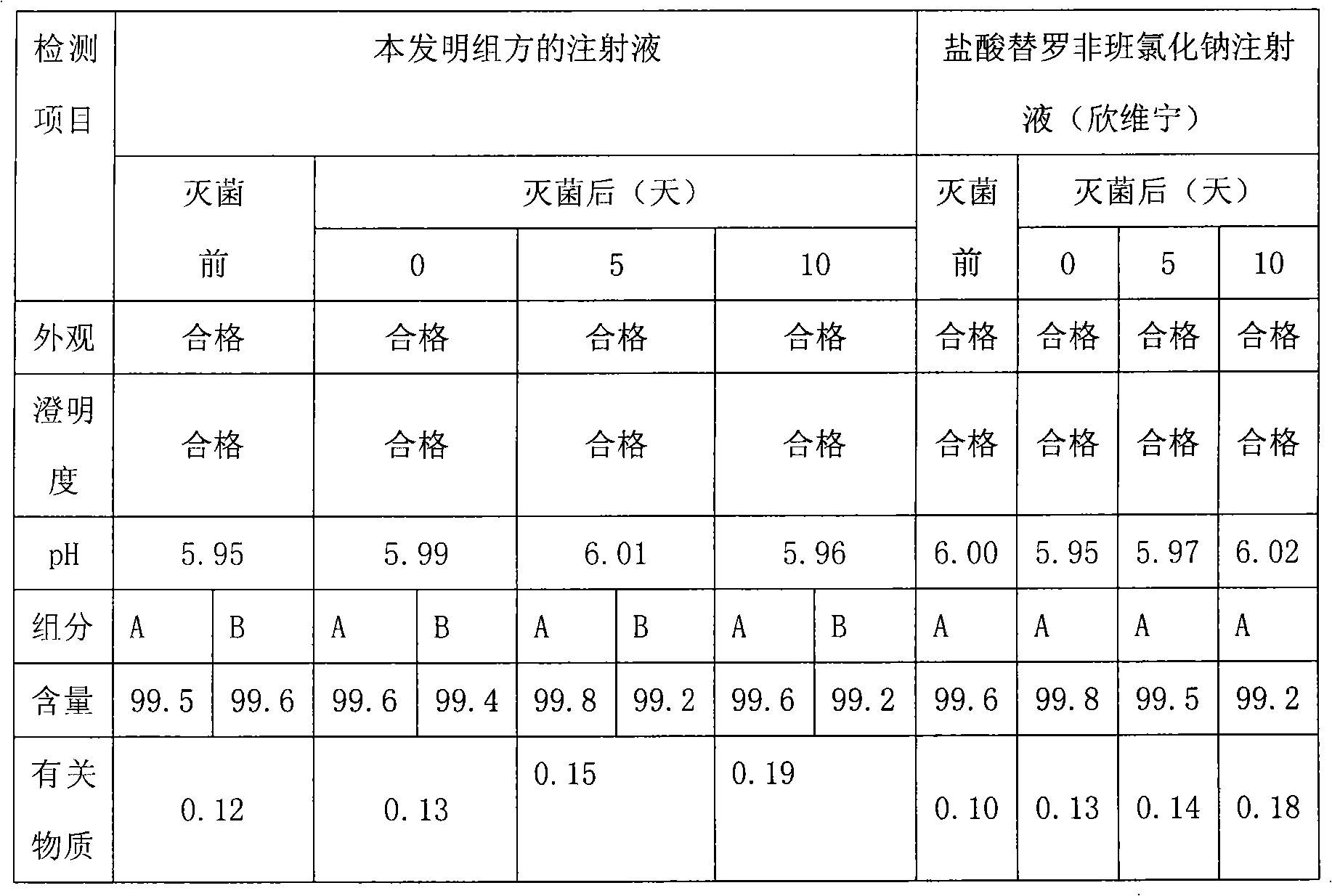
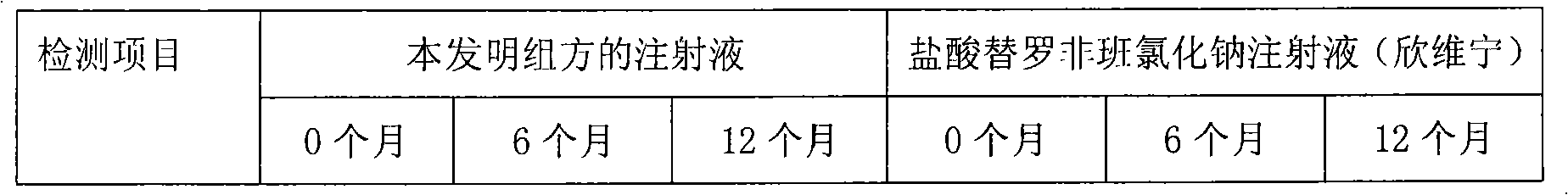
[0030] Drug compatibility and stability of embodiment 1 tirofiban and arginine aspirin injection
[0031] We combine tirofiban and arginine aspirin to prepare tirofiban-arginine aspirin injection, specification: 100ml / bottle, each bottle contains tirofiban hydrochloride 5.618mg (in the form of anhydrous tirofiban Class 5mg), arginine aspirin 393.3mg (equivalent to acetylsalicylic acid 200mg) and sodium chloride 0.9g.
[0032] prescription:
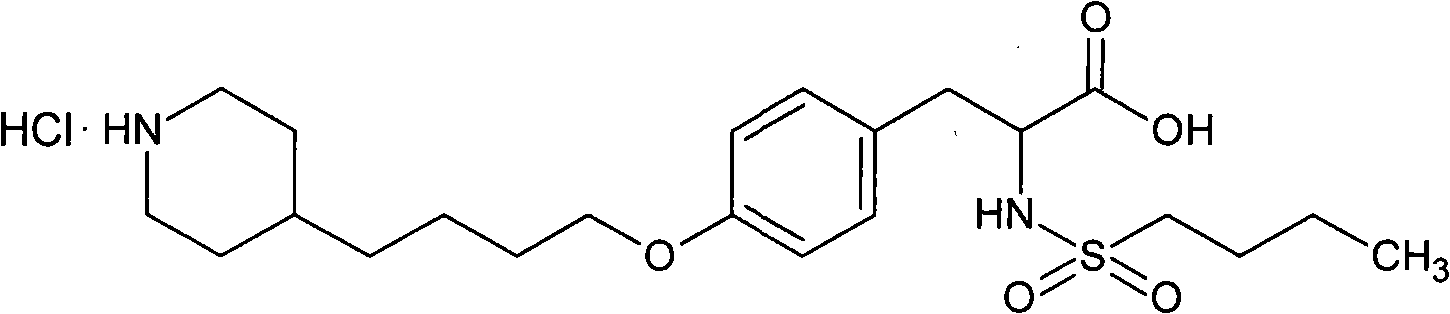
[0033] Tirofiban Hydrochloride 56.18mg
[0036] Water for injection to 1000ml
[0037]
[0038] A total of 10 bottles of injection were made
[0039] Preparation Process:
[0040] Weigh sodium chloride according to the prescription amount, add it to 100ml of water for injection, stir until completely dissolved; weigh 0.3% activated carbon in solution, stir well, heat and boil for 15 minutes, after cooling, filter out the activated carbon; a...
Embodiment 2
[0051] Embodiment 2 The injection of prescription of the present invention is to rat right carotid artery-vein bypass thrombosis experiment experimental drug:
[0052] normal saline
[0054] Tirofiban
[0055] Tirofiban-lysine aspirin injection (divided into large, medium and small dose groups)
[0056] Experimental animals: 60 Wistar rats, half male and half male, body weight 250±10g.
[0057] Rats were randomly divided into 6 groups, 10 in each group. Drug dosage is (A) blank control (0.9% sodium chloride solution), (B) lysine aspirin group (240.0mg·kg -1 ), (C) tirofiban group (8.0mg·kg -1 ), (D) tirofiban-lysine aspirin group (2.0 / 160.0mg·kg -1 ), (E) tirofiban-lysine aspirin group (4.0 / 240.0mg·kg -1 ), (F) tirofiban-lysine aspirin group (8.0 / 320.0mg·kg -1 ).
[0058] experimental method:
[0059] Animals in each group were administered intraperitoneally according to the above dose, and the blank group was given the same amount of normal s...
Embodiment 3
[0064] Example 3 Tirofiban-Arginine Aspirin Injection Measures Thrombosis Time in Rats
[0065] experimental method:
[0066] Rats were taken, and the administration was the same as before, once a day for 3 consecutive days, and 30 minutes after the last administration, chloral hydrate (350 mg·kg -1 ) anesthetized, fixed in the supine position, separated the right common carotid artery about 15mm, placed the stimulating electrode and temperature sensor probe of the in vivo thrombosis instrument on the artery, stimulated the blood vessel with 2mA current to damage the arterial endothelium, recorded the result of thrombus in the arterial lumen The time it takes to block blood flow is called the thrombosis time. The measurement results are shown in Table 4:
[0067] The impact of table 4 on the time of thrombus formation in rats (X±S, n=10)
[0068]
[0069] Compared with normal saline group, *P<0.05, **P<0.01
[0070] The results showed that both group E and group F conta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com