Method for synthesis epoxy cyclohexane with titanium molecular sieve catalysis
A technology of titanium-silicon molecular sieve and epoxycyclohexane, which is applied in chemical recovery, organic chemistry, etc., can solve the problems of low yield of epoxycyclohexane, catalyst loss and denaturation, and insufficient production, and achieve simple reaction process , high selectivity, and the effect of meeting the requirements of technology and economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
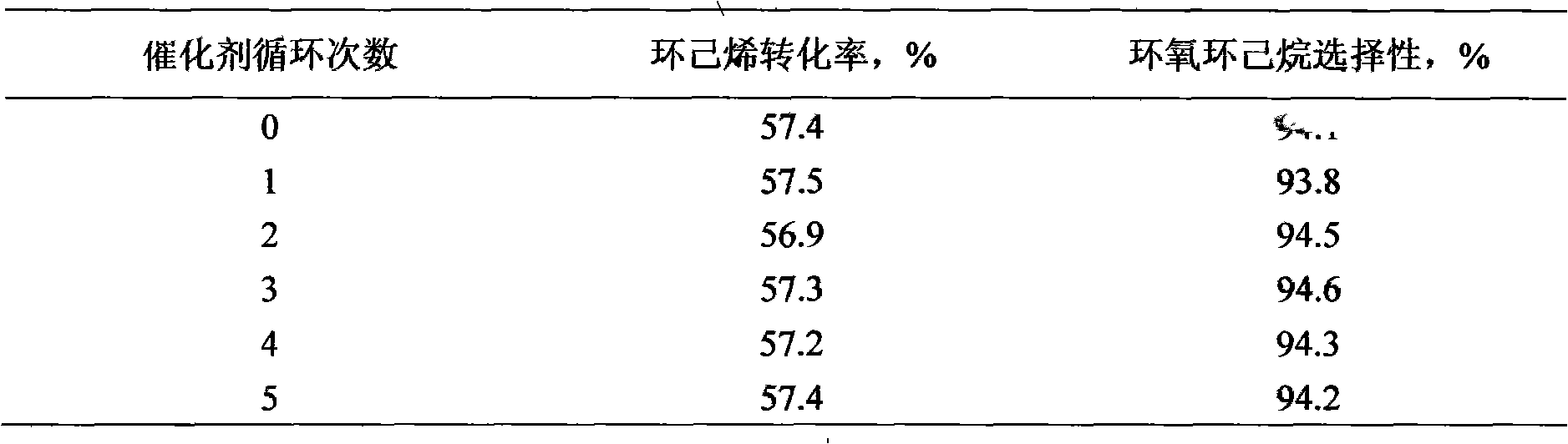
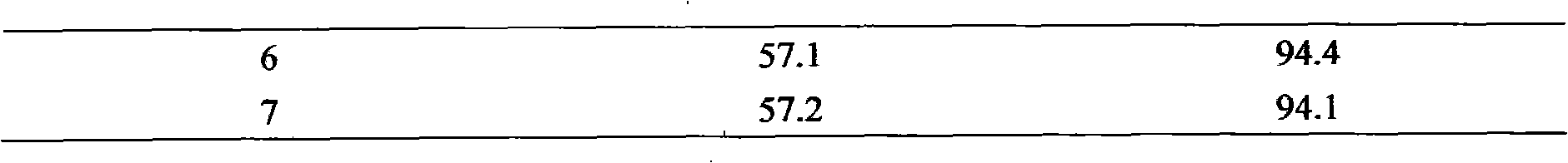
[0013] Take 1.0 mol of cyclohexene, 200 ml of methanol and 5.0 g of TS-1 molecular sieve (with a particle size of 0.02 μm to 0.1 μm), and add 0.7 mol of 10% hydrogen peroxide aqueous solution (w / w) dropwise at 35° C. After the dropwise addition, the reaction was carried out at the same temperature for another 3 hours, the conversion rate of cyclohexene was 64.0%, the utilization rate of hydrogen peroxide was 91%, and the selectivity of epoxycyclohexane was 92.6%. Methanol, unreacted cyclohexene and product epoxycyclohexane with a content of 99.7% are obtained by rectification. The methanol and cyclohexene and the unsteamed catalyst are used for the next reaction.
Embodiment 2
[0015] Take 1.5 mol of cyclohexene, 500 ml of acetonitrile and 4.0 g of TS-1 molecular sieve (with a particle size of 0.1 μm to 0.8 μm), and add 0.75 mol of 35% aqueous hydrogen peroxide (w / w) dropwise at 70° C. After the dropwise addition, another reaction was performed at the same temperature for 1 hour, the conversion rate of cyclohexene was 47.5%, the utilization rate of hydrogen peroxide was 95%, and the selectivity of epoxycyclohexane was 89.7%. Through filtration, 3.9g of the catalyst on filter paper were dried; the filtrate obtained acetonitrile and unreacted cyclohexene and product epoxycyclohexane with a content of 99.5% by rectification. The acetonitrile and cyclohexene and the recovered catalyst are used for the next reaction.
Embodiment 3
[0017] Take 1.5 mol of cyclohexene, 500 ml of ethyl acetate and 4.0 g of TS-1 molecular sieve, and add 0.75 mol of 35% aqueous hydrogen peroxide solution (w / w) dropwise at 70°C. After the dropwise addition, the reaction was carried out at the same temperature for another hour, the conversion rate of cyclohexene was 48.0%, the utilization rate of hydrogen peroxide was 96%, and the selectivity of epoxycyclohexane was 87.0%. Through filtration, 3.9 g of the catalyst on filter paper were dried; the filtrate was obtained through rectification to obtain ethyl acetate, unreacted cyclohexene and product epoxycyclohexane with a content of 99.5%. Ethyl acetate and cyclohexene and the recovered catalyst are used for the next reaction.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com