Target-specificity dual-mutant amalgamation protein
A fusion protein, target-specific technology, applied in the field of target-specific double mutant fusion protein, can solve problems such as toxicity and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
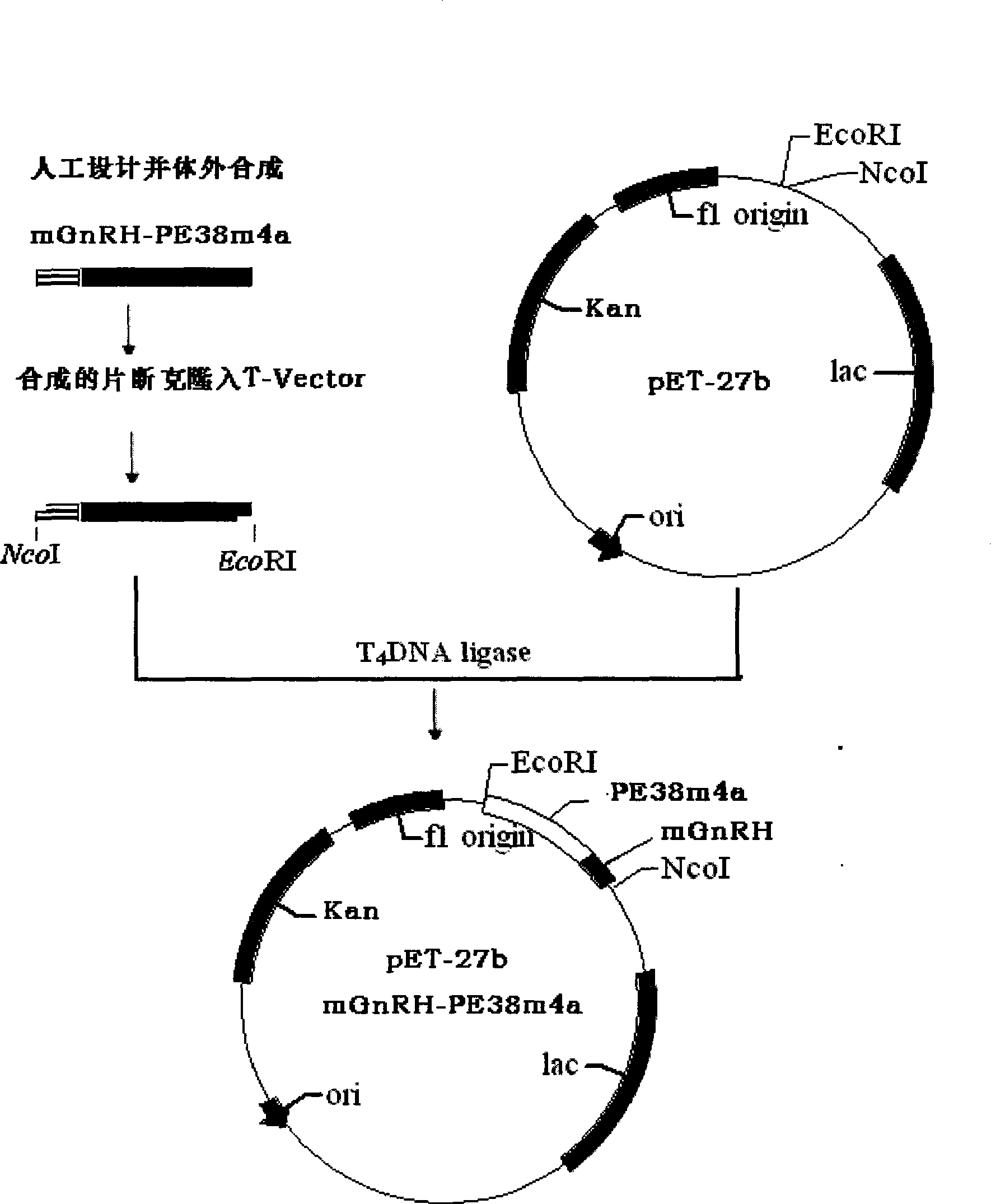
[0050] Example 1: Preparation of mGnRH-PE38m4a fusion protein
[0051] A. Construction and identification of recombinant expression plasmids
[0052] a. Preparation of mGnRH-PE38m4a double-stranded gene: refer to the existing literature to design and artificially synthesize the double-stranded nucleotide sequence of mGnRH-PE38m4a shown in SEQ ID NO. ). at T 4 In the presence of ligase (Promega), it was directly cloned into PGEM-T vector, and transformed into Escherichia coli JM105 to obtain an engineering strain containing PGEM-T / mGnRH-PE38m4a plasmid.
[0053] b. Preparation of mGnRH-PE38m4a expression plasmid: extract PGEM-T / mGnRH-PE38m4a plasmid, and use NcoI and EcoRI endonuclease double digestion, collect mGnRH-PE38m4a gene fragment, in T 4 In the presence of ligase (Promega), it was directly cloned into the pET27 vector digested with the same restriction enzymes, and transformed into Escherichia coli JM105 to obtain an engineering strain containing the pET27-mGnRH-PE3...
Embodiment 2
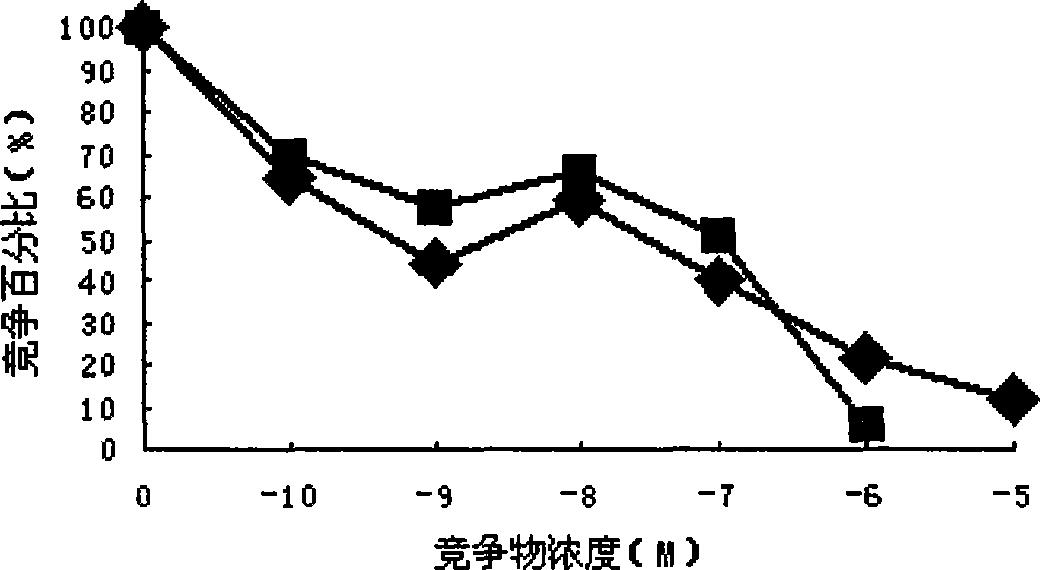
[0059] Example 2: 125 Target specificity and biological activity analysis of I-labeled native GnRH and mGnRH-PE38m4a fusion proteins
[0060] A. Binding ability test:
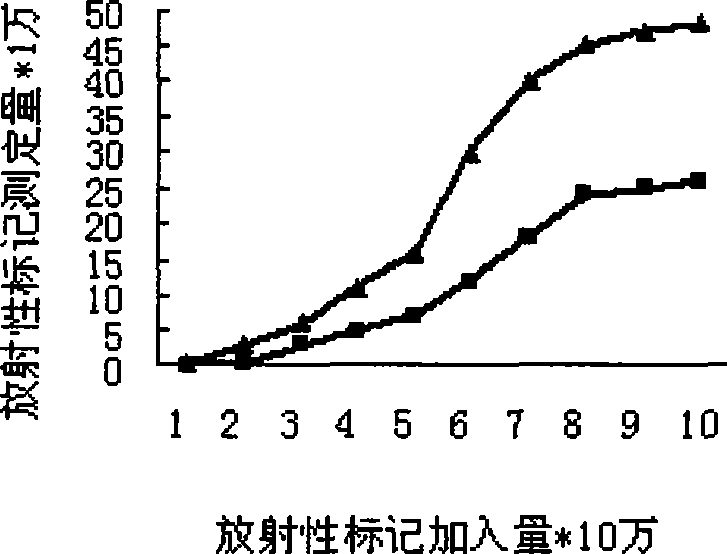
[0061] a. GnRH and mGnRH-PE38m4a 125 Mark I: Weigh 1mg Lodogen and dissolve it in 0.5ml chloroform, add 50μl (100μg) to the bottom of the test tube, blow dry with nitrogen, add 0.4ml of peptide or protein semi-finished product without protective agent, add Na 125 I5mCi was reacted at room temperature for 12 minutes, and the reaction process was shaken continuously to make it fully reacted.
[0062] 125 The I-labeled mixture was separated and purified using a Sepharyl S-200HR gel column (1×50 cm), and the tubes were collected, and the tube with the highest radioactive intensity was used for testing.
[0063] b. Cancer cell and normal cell culture: Use RPMI-1640 complete medium in 5% CO 2 , 37 ° C monolayer adherent culture and counting. Add the same CPM to each cell well 125 I labeled GnRH and mGnRH-PE38m...
Embodiment 3
[0070] Embodiment 3: cytotoxicity test:
[0071] Quantitative samples were filtered and sterilized, and different amounts of samples were added to each cell well according to the equal dilution method, so that the total volume was 100 μl, 5% CO 2 , cultured at 37°C for 12 hours, and added 100 μl of MTT staining reagent to each well of the culture plate, 5% CO 2 , continue culturing for 4 hours at 37°C, measure the absorbance value at 490nm wavelength, and calculate the concentration of 50% cell death (IC 50 ), while using natural Pseudomonas aeruginosa exotoxin A (PEA, Sigma Company) as a control to compare the activity changes of the fusion toxin and natural PEA. The results are shown in Table 1.
[0072] Table 1: IC of purified mGnRH-PE38m4a and native PEA on tumor and normal cells 50
[0073]
[0074] Table 1 shows the cytotoxicity of purified mGnRH-PE38m4a and native PEA on certain tumor cells and certain normal cell cultures. IC 50 Values refer to the concentra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com