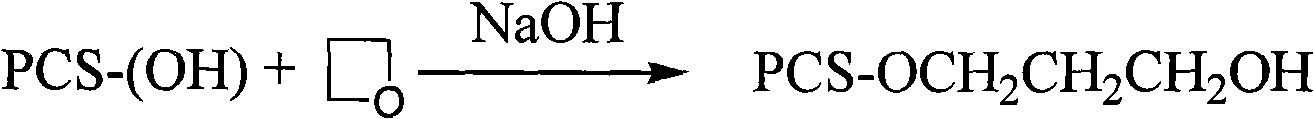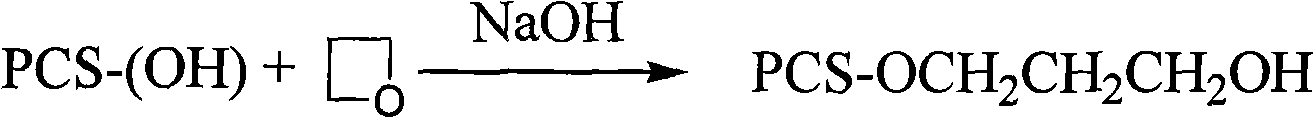Synthesis of substituted pachyman
A technology of polysaccharides and synthesis methods of Poria cocos, applied in the direction of drug combinations, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of difficulty in suction filtration or centrifugal separation, large consumption of organic solvents, low yield, etc., and achieve easy operation and processing , Simple and time-saving operation, and the effect of improving the degree of product substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 in NaOH aqueous ethanol solution, the synthesis of carboxymethylpachyran
[0029] Add solid NaOH (3.0g, 75mmol) and 85% ethanol (20mL) to a 50mL single-necked round-bottom flask successively to dissolve NaOH, then add 1.0g of Poria cocos powder, alkalinize for 0.5h under the action of ultrasonic waves, and then add chloroacetic acid (1.17 g, 12.4mmol), reacted at 50°C for 50 minutes under the continued action of ultrasound, separated into layers, poured off the supernatant, dissolved the paste in the lower layer in a small amount of alkali, neutralized with glacial acetic acid, precipitated with alcohol, filtered with suction, washed, Freeze-dried to obtain a white powder with a measured degree of substitution of 0.68.
Embodiment 2
[0030] Embodiment 2 in NaOH urea aqueous solution, the synthesis of methylpachyran
[0031] Add 1.5M NaOH / 0.5M urea aqueous solution (18.5mL) into a 50mL single-necked round-bottom flask, then add 1.0g of poria cocos powder, alkalinize under ultrasonication for 1.0h, then add dimethyl sulfate (1.75g, 13.9mmol), and ultrasonically Continue to react at 60°C for 60 minutes, neutralize with glacial acetic acid, filter with suction, wash, and freeze-dry to obtain a white powder with a measured substitution degree of 0.51.
Embodiment 3
[0032] Embodiment 3 in NaOH urea aqueous solution, the synthesis of hydroxyethylpachyran
[0033] Add 1.5M NaOH / 0.5M urea aqueous solution (25mL) into a 50mL single-necked round-bottom flask, then add 1.0g of Poria cocos powder, alkalinize for 1.0h under the action of ultrasonic waves, then slowly drop 2-chloroethanol (1.0g, 12.4mmol) into In the reaction system, it was reacted at 60°C for 90 minutes under the action of ultrasound, neutralized with glacial acetic acid, filtered with suction, washed, and freeze-dried to obtain a white powder with a measured substitution degree of 0.42.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com