Room-temperature decomposition ozone catalysis material and preparation method thereof
A catalytic material, manganese oxide technology, applied in the fields of catalysis and environmental science, can solve problems such as poor moisture resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
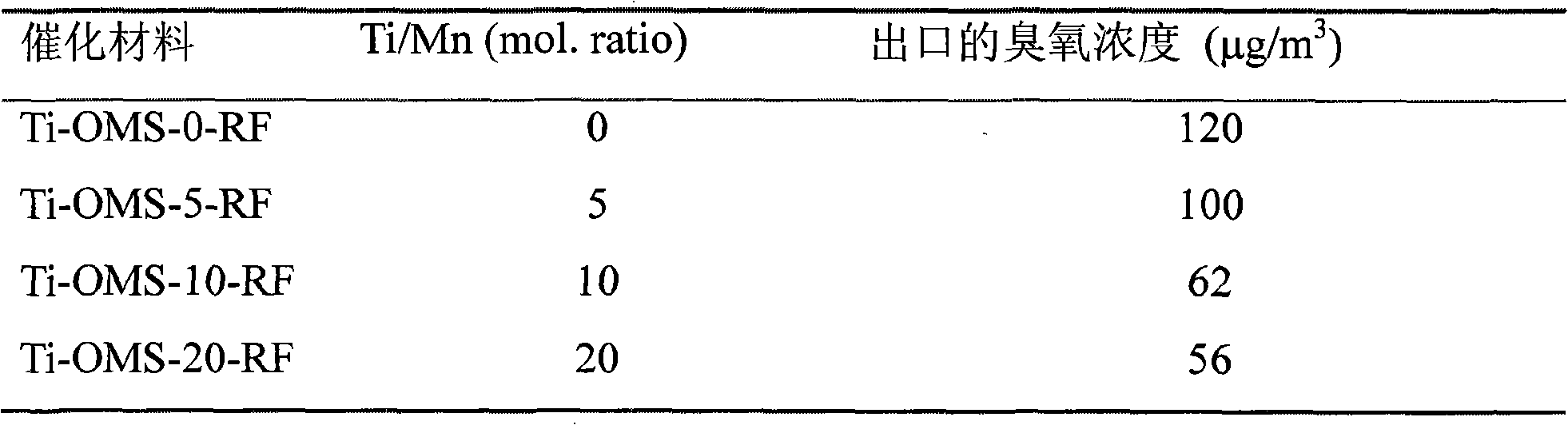
[0031] Titanium-doped manganese oxide molecular sieve powders were synthesized by potassium permanganate (KMnO 4 ) and potassium persulfate (K 2 S 2 o 8 ) in nitric acid solution to oxidize manganese sulfate (MnSO 4 ·H 2 O), while adding titanium sulfate (Ti(SO 4 ) 2 ), the Ti / Mn molar ratio is 0 to 0.20, and the resulting black precipitate is vigorously stirred and refluxed at 100°C for 24h, filtered, washed, dried at 110°C for 12h, and then calcined at 500°C for 6 hours to obtain the oxidation of doped titanium. Manganese molecular sieve powder, respectively denoted as Ti-OMS-0-RF, Ti-OMS-5-RF, Ti-OMS-10-RF and Ti-OMS-20-RF, where Ti-OMS means titanium-doped manganese oxide Molecular sieve, the number indicates Ti / Mn total The molar ratio percentage, RF represents the reflux synthesis method, ie (Refluxing).
[0032] XRD analysis and HRTEM analysis indicated that the material was Hollandite type manganese oxide.
[0033] The performance tests of the catalytic materi...
Embodiment 2
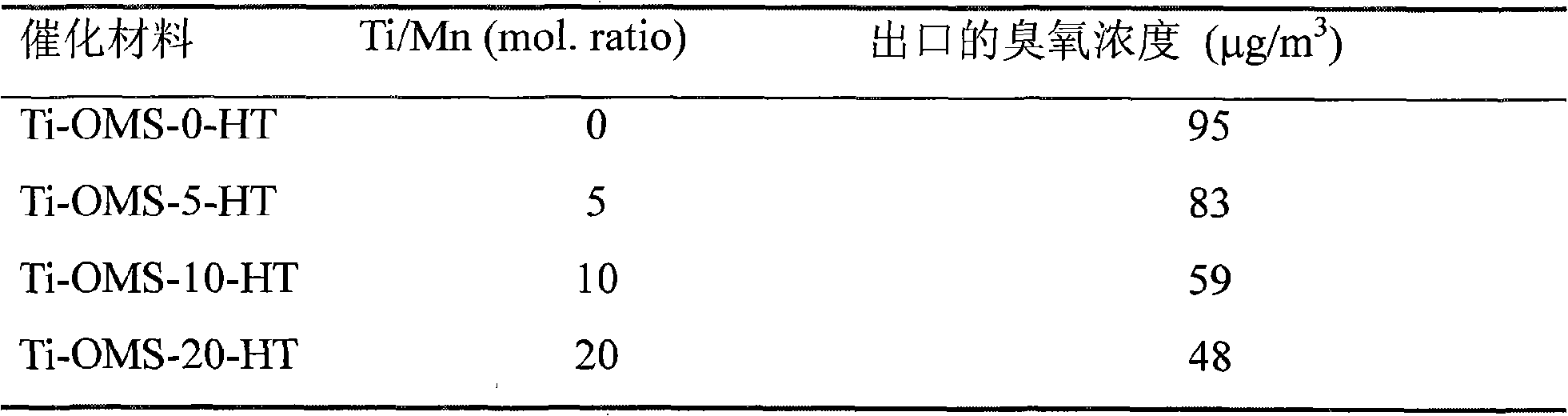
[0035] Titanium-doped manganese oxide molecular sieve powders were synthesized by potassium permanganate (KMnO 4 ) and potassium persulfate (K 2 S 2 o 8 ) in nitric acid solution to oxidize manganese sulfate (MnSO 4 ·H 2 O), while adding titanium sulfate (Ti(SO 4 ) 2 ), then transferred to an autoclave, sealed and kept at a constant temperature of 160°C for 48h, after natural cooling, filtered, washed, dried at 110°C for 24h, and then roasted at 500°C to obtain titanium-doped manganese oxide molecular sieve powder, respectively calculated as Ti -OMS-0-HT, Ti-OMS-5-HT, Ti-OMS-10-HT and Ti-OMS-20-HT, where Ti-OMS represents titanium-doped manganese oxide molecular sieve, and the numbers represent Ti / Mn total The molar ratio percentage, HT represents the hydrothermal synthesis method, ie (Hydrothermal).
[0036]XRD analysis and HRTEM analysis showed that the material was Hollandite manganese oxide, and titanium ions were located on the framework of manganese oxide molecula...
Embodiment 3
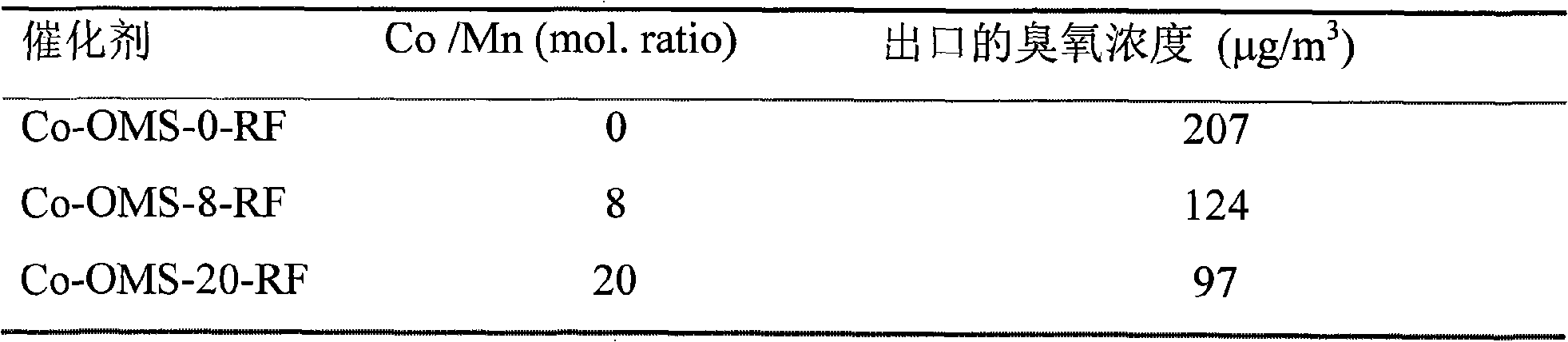
[0039] Cobalt-doped manganese oxide molecular sieve powders were synthesized by potassium permanganate (KMnO 4 ) and potassium persulfate (K 2 S 2 o 8 ) in nitric acid solution to oxidize manganese sulfate (MnSO 4 ·H 2 O), while adding cobalt nitrate (Co(NO 3 ) 2 ·6H 2 O), the Co / Mn molar ratio is 0 to 0.20, the resulting black precipitate is vigorously stirred and refluxed at 100°C for 24h, filtered, washed, dried at 110°C for 12h, and then calcined at 500°C for 6 hours to obtain doped cobalt Manganese oxide molecular sieve powder, respectively counted as Co-OMS-0-RF, Co-OMS-8-RF and Co-OMS-20-RF, where Co-OMS means titanium-doped manganese oxide molecular sieve, and the number means Co / Mn total The molar ratio percentage, RF represents the hydrothermal synthesis method, ie (Refluxing).
[0040] XRD analysis and HRTEM analysis showed that the material was Hollandite manganese oxide, and cobalt ions were located on the framework of manganese oxide molecular sieve.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com