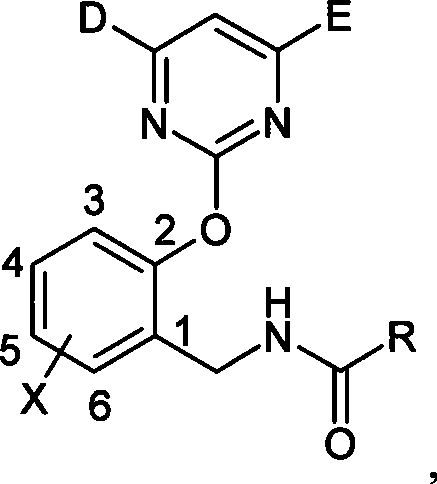
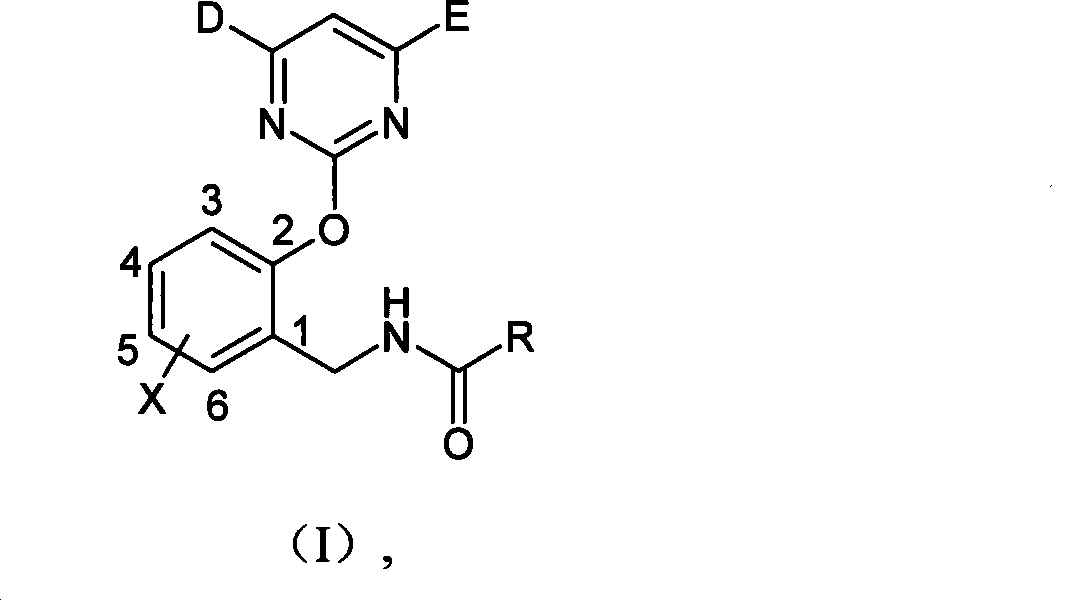
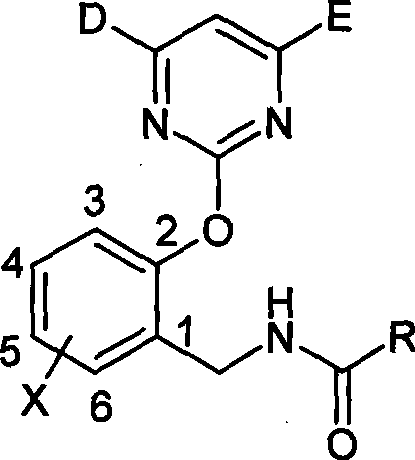
N-(2-pyrimidine oxygen based) benzyl amide compounds, preparation method and uses thereof
A technology of benzylamide and pyrimidinyloxy, applied in the field of agrochemical herbicides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of N-(2-pyrimidinyloxy)benzylamides
[0049] Take the synthesis of I-5 as an example: (o-chlorobenzoyl chloride)
[0050] Add 9.92g (81.3mmol) salicylaldehyde, 5.93g (85.3mmol) hydroxylamine hydrochloride and 7.51g (89.4mmol) sodium bicarbonate, ethyl acetate 100ml and about 8ml water in a 250mL three-necked flask, at room temperature, stir about overnight. Filtered, washed with water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain 10 g with a yield of 89.7%.
[0051] Reduction to benzylamine: Dissolve 13.7g (0.1mol) of the product in 20mL of anhydrous methanol, pour it into a 250ml three-neck flask, add 40.8g (0.624mol) of zinc powder while stirring, and finally add 28.9g (0.458mol) of ammonium formate methanol solution. React overnight at room temperature. Adjust the pH to 3, filter, and adjust the filtrate to neutral with ammonia water, and a light yellow solid appears. Filter and dry. Obtained 7.9...
Embodiment 2
[0062] The synthesis of I-1, the detailed experimental steps are the same as in Example 1: (1) Reaction of 2-hydroxybenzylamine and isobutyryl chloride: feed 6 mmol of isobutyryl chloride to obtain 1.065 g of the product, with a yield of 92%. (2) Condensation with 4,6-dimethoxy-2-thiamphenicol pyrimidine, using acetonitrile as solvent, synthesized at 40-50°C. The pure product was obtained by column chromatography (petroleum ether: ethyl acetate = 3:1), with a yield of 90%.
[0063] solid
[0064] m.p.: 118-120°C
[0065] 1 H NMR (300MHz, CDCl 3 / TMS): δ 7.37-6.85 (4H, m, CH), 6.634 (1H, s, NH), 5.768 (1H, s, CH), 4.61-4.63 (2H, d, J=5.1Hz), 4.20 ( 1H, m, CH), 3.81 (6H, s, OCH 3 ), 2.52 (6H, s, CH 3 , J=7.2Hz)
[0066] MS (EI): 332 (M + , 100), 354 (M+Na + , 20)
[0067] E.A. for C 17 h 21 N 3 o 4
[0068] Calcd: C 61.62, H 6.39, N 12.68
[0069] Found: C 61.76, H 6.54, N 12.76
Embodiment 3
[0071] The synthesis of I-2, the detailed experimental steps are the same as in Example 1: (1) Reaction of 2-hydroxy-6-chlorobenzylamine with isobutyryl chloride: 6 mmol of isobutyryl chloride was fed to obtain 1.294 g of the product, with a yield of 95%. (2) Condensation with 4,6-dimethoxy-2-thiamphenicol pyrimidine, using acetonitrile as solvent, synthesized at 40-50°C. The pure product was obtained by column chromatography (petroleum ether: ethyl acetate = 3:1), with a yield of 85%.
[0072] solid
[0073] m.p.: 140-142°C
[0074] 1 H NMR (300MHz, CDCl 3 / TMS): δ 7.43-7.05 (3H, m, CH), 6.63 (1H, s, NH), 5.87 (1H, s, CH), 4.61-4.63 (2H, d, J=5.1Hz), 4.20 ( 1H, m, CH), 3.81 (6H, s, OCH 3 ), 2.52 (6H, s, CH 3 , J=7.2Hz)
[0075] MS(EI): 366(M + , 100), 388 (M+Na + , 20)
[0076] E.A. for C 17 h 20 ClN 3 o 4
[0077] Calcd: C 55.82, H 5.51, N 11.49
[0078] Found: C 55.90, H 5.65, N 11.65
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com