Pyrazoline pyridine-fluorescent coumarin dye derivant, synthesis and uses thereof
A pyrazoline pyridine and fluorescent dye technology, applied in the field of laser dyes and fluorescent dyes, can solve problems such as laser formation and destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) Synthesis of N, N-diethylcoumarin aldehyde (synthetic method according to J.Med.Chem.2004, 47, 6349)
[0078]
[0079] Add 2mL of phosphorus oxychloride to a 50mL three-necked flask, slowly add 2mL of freshly steamed dimethylformamide (DMF) dropwise under nitrogen protection, and complete the dropwise addition within 30 minutes. The temperature of the reaction solution is controlled at 20-50°C. 10 mL of a DMF solution in which 1.50 g of 7-N,N-diethylaminocoumarin (6.91 mmol) was dissolved was added dropwise thereto. After the dropwise addition was completed, the reaction solution was continued to react at 60° C. for 12 hours. The reaction solution was poured into 100 mL of ice water, and then the pH value of the reaction solution was adjusted with a 20 wt % NaOH aqueous solution, and a large amount of red solids were precipitated. The crude product was filtered, washed several times with water, dried in vacuo, and then recrystallized with absolute ethanol to obt...
Embodiment 2
[0086] Synthesis of Coumarin Derivative (II)-2
[0087]
[0088] The synthesis of N, N-diethylcoumarin aldehyde and intermediate (III)-1 is the same as in Example 1.
[0089] Dissolve 1.63g (0.005mol) of compound (III)-1 and 0.61g (0.005mol) of p-methylphenylhydrazine in 30mL of ethanol, heat and reflux at 90°C for 6 hours, after cooling, a yellow solid precipitates, which is recrystallized after filtration to obtain 1.12 g coumarin derivative (II)-2, yield 54.4%.
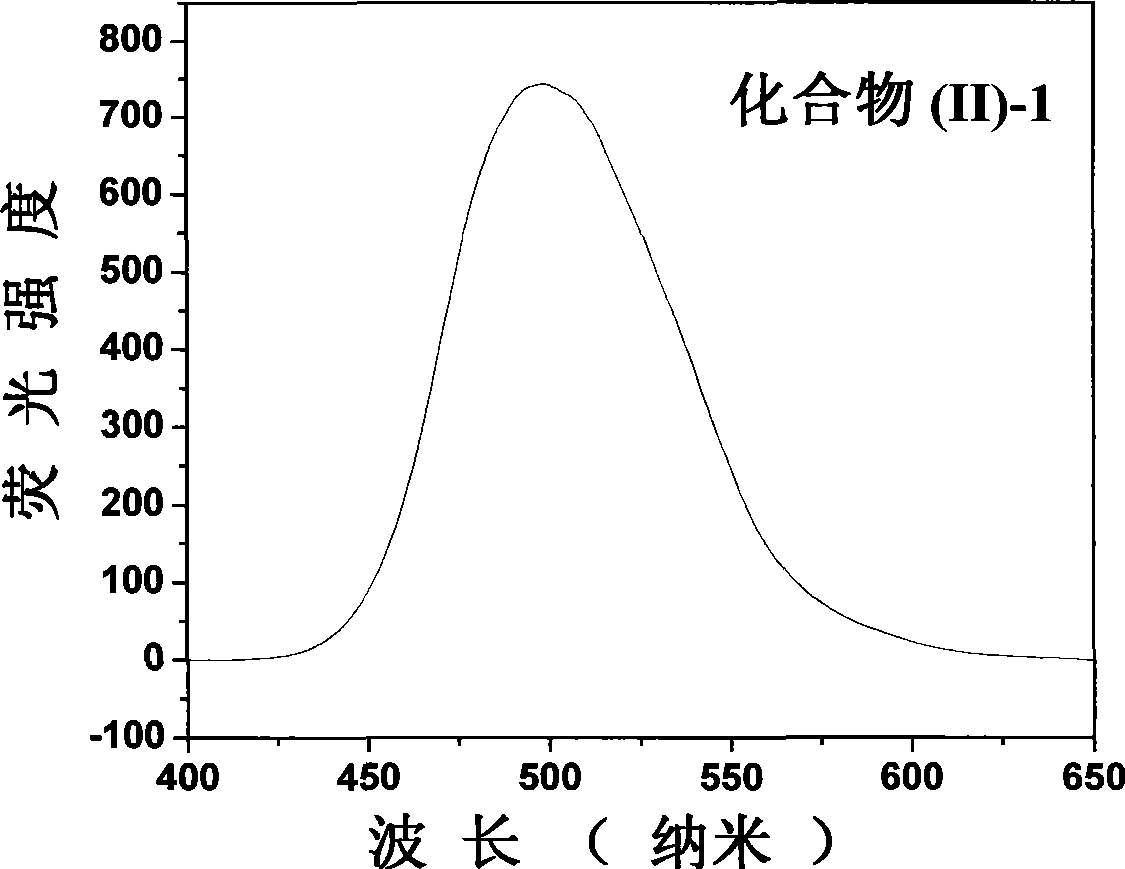
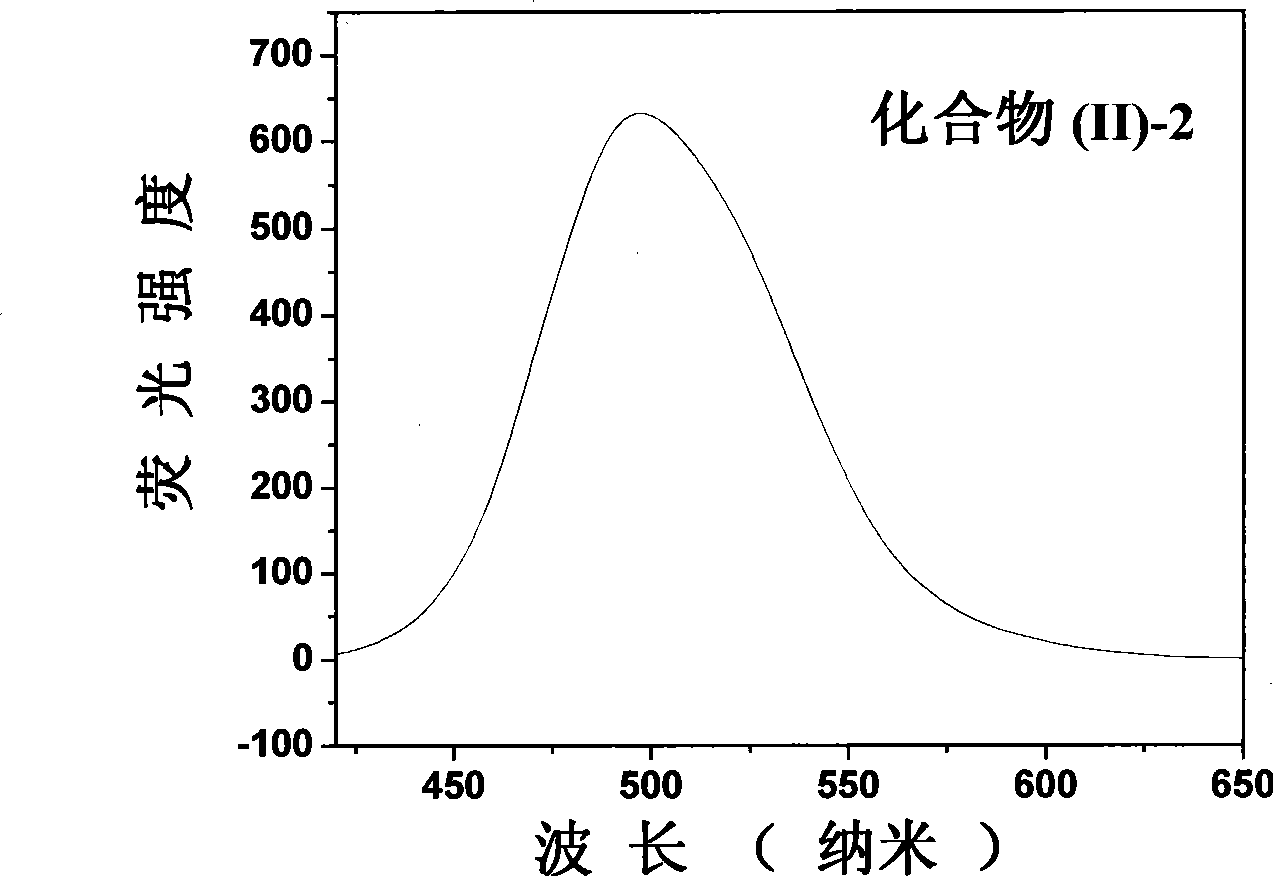
[0090] EI-MS, m / e, 413.2[M+1] + .λ ab. max / nm=390nm, λ em max / nm=497nm, Φ f e = 0.97.
Embodiment 3
[0092] Synthesis of Coumarin Derivative (II)-3
[0093]
[0094]
[0095] The synthesis of N, N-diethylcoumarin aldehyde is the same as in Example 1.
[0096] Dissolve 2.45g (0.01mol) of N,N-diethylcoumarin aldehyde and 1.63g (0.01mol) of benzoylacetamide in 30mL of ethanol, add 0.5mL of triethylamine, and heat to reflux at 90°C for 8 hours. After cooling, a yellow solid was precipitated, and 2.16 g of (III)-2 intermediate was obtained by filtration, with a yield of 55.7%. Compound (III)-2 can be directly used in the next reaction without purification.
[0097] Dissolve 1.94g (0.005mol) of compound (III)-2 and 0.54g (0.005mol) of phenylhydrazine in 30mL of ethanol, and heat to reflux at 90°C for 6 hours. After cooling, a yellow solid precipitates, which is filtered and recrystallized to obtain 1.27g of coumarin The yield of prime derivative (II)-3 is 55.2%.
[0098] EI-MS, m / e, 461.2[M+1] + .λ ab. max / nm=392nm, λ em max / nm=515nm, Φ f e = 0.99.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com