Method for aluminum lithium alloy preparation by fused salt electrolysis process
An aluminum-lithium alloy, molten salt electrolysis technology, applied in the field of material science, can solve the problems of increasing the volatilization loss of lithium chloride, shortening the service life of the electrolytic cell, personal and environmental damage, etc., and achieves stable and easy control of the electrolysis process and energy consumption. Reduced, easy to operate and manage effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
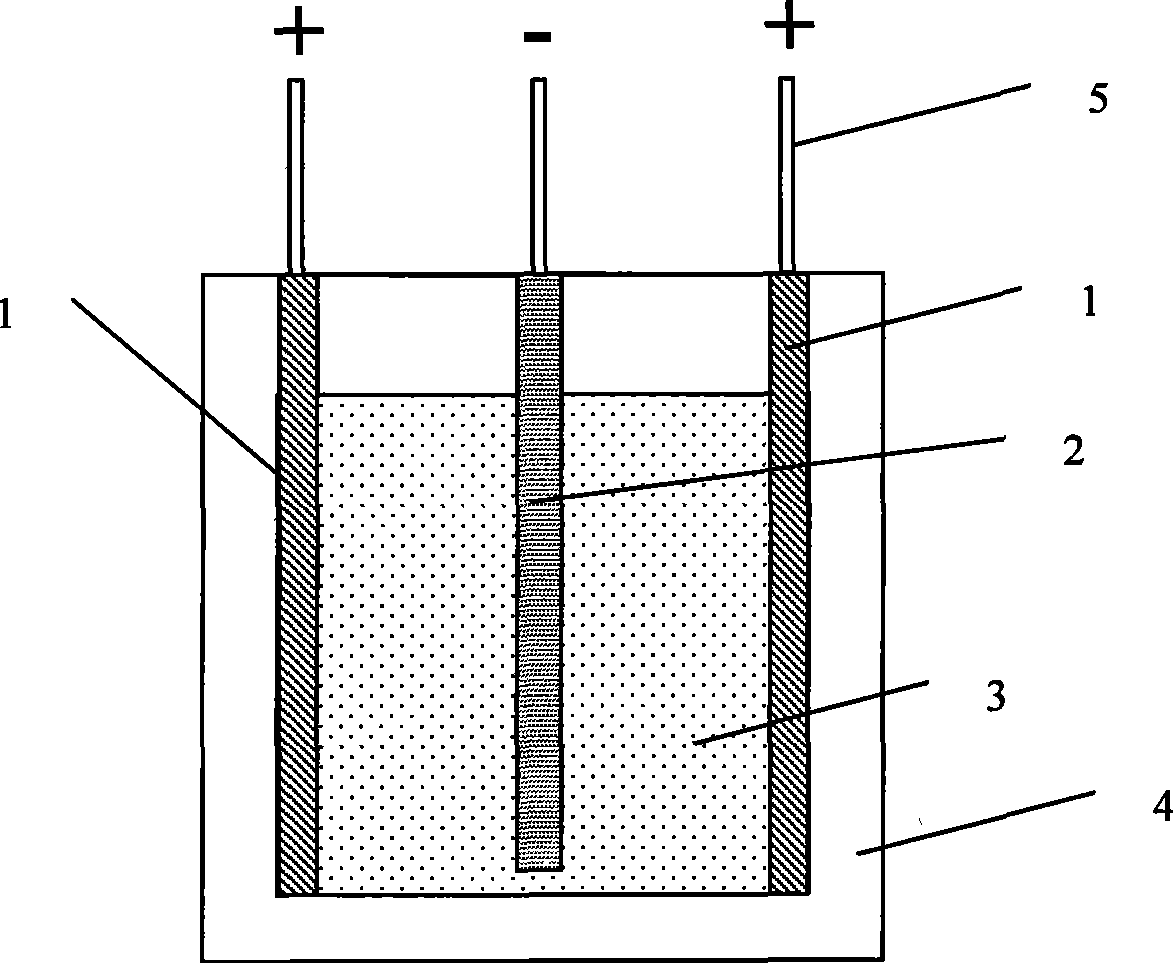
[0030] Using an electrolytic cell device such as figure 1 As shown, the aluminum plate cathode 2 is placed in the middle of the two graphite anodes 1, the electrolytic cell body 4 is made of stainless steel, and the corundum insulating plate is laid on the bottom, and the electrolytic cell adopts an external heating method; the anode and the cathode are connected to the power supply through the electrode lead 5, and the electrolysis Mixed salt 3 is placed inside the tank body 4 .
[0031] Among them, the area of the cathode aluminum plate is 200cm 2 , with a thickness of 0.5cm and a mass of 135g.
[0032] Potassium carbonate and lithium carbonate are mixed, and the mixing ratio is that lithium carbonate accounts for 26% of the total mass of the mixed carbonate, and potassium carbonate accounts for 74% of the total mass of the mixed carbonate.
[0033] Put 1000g of mixed carbonate in the electrolytic cell, heat the electrolytic cell containing the mixed carbonate to 550°C, ...
Embodiment 2
[0037] Adopt electrolyzer device with embodiment 1, wherein the cathode aluminum plate area is 200cm 2 , with a thickness of 0.5cm and a mass of 135g.
[0038] Potassium carbonate and lithium carbonate are mixed, and the mixing ratio is that lithium carbonate accounts for 47% of the total mass of the mixed carbonate, and potassium carbonate accounts for 53% of the total mass of the mixed carbonate.
[0039] Put 1000g of mixed carbonate in the electrolytic cell, heat the electrolytic cell containing the mixed carbonate to 490°C, the mixed carbonate will melt, continue heating to 530°C, and then energize the two poles to start electrolysis, the current density during electrolysis 0.1A / cm 2 After the electrolysis is completed, the cathode mixed with lithium metal is taken out, and then put into a new cathode aluminum plate to continue the electrolysis. The use time of each new aluminum plate in the electrolytic cell is 2.1 to 2.5 hours.
[0040] When the concentration of the l...
Embodiment 3
[0043] Adopt electrolyzer device with embodiment 1, wherein the cathode aluminum plate area is 200cm 2 , with a thickness of 0.5cm and a mass of 135g.
[0044] Potassium carbonate and lithium carbonate are mixed, and the mixing ratio is that lithium carbonate accounts for 52% of the total mass of the mixed carbonate, and potassium carbonate accounts for 48% of the total mass of the mixed carbonate.
[0045] Put 1000g of mixed carbonate in the electrolytic cell, heat the electrolytic cell containing the mixed carbonate to 550°C, the mixed carbonate will melt, continue heating to 580°C, and then energize the two poles to start electrolysis, the current density during electrolysis 0.1A / cm 2After the electrolysis is completed, the cathode mixed with lithium metal is taken out, and then put into a new cathode aluminum plate to continue the electrolysis. The use time of each new aluminum plate in the electrolytic cell is 1.4 to 2 hours.
[0046] When the concentration of lithium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com