Chemical luminescence immune analysis quantitative measuring reagent kit for IV type collagen and preparation method thereof
A collagen and chemiluminescent technology, applied in the field of immunoanalysis medicine, can solve the problems of expensive instruments, radioactive contamination, narrow linear range, etc., and achieve the effects of ensuring sensitivity, easy operation and production, and improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
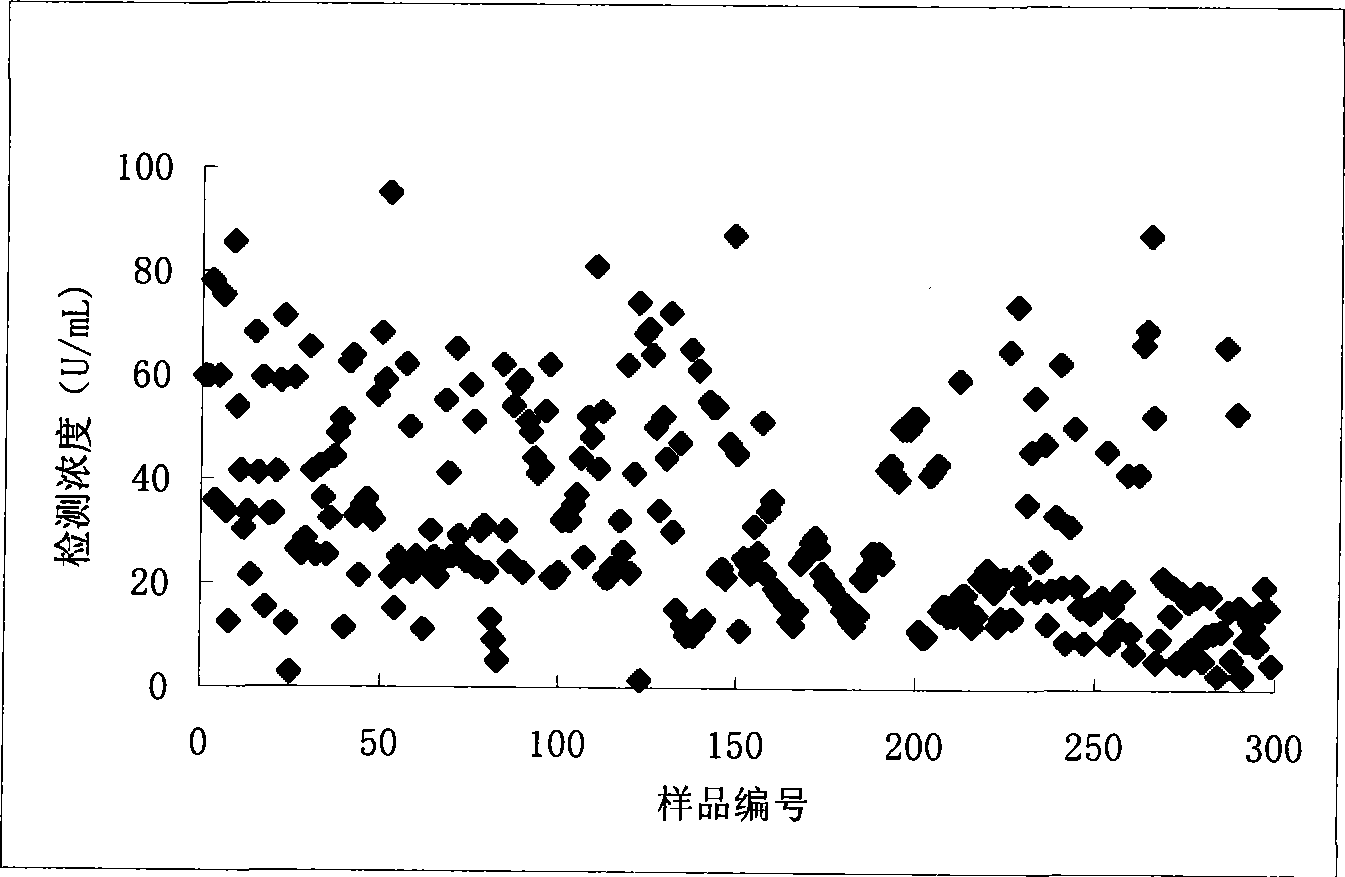
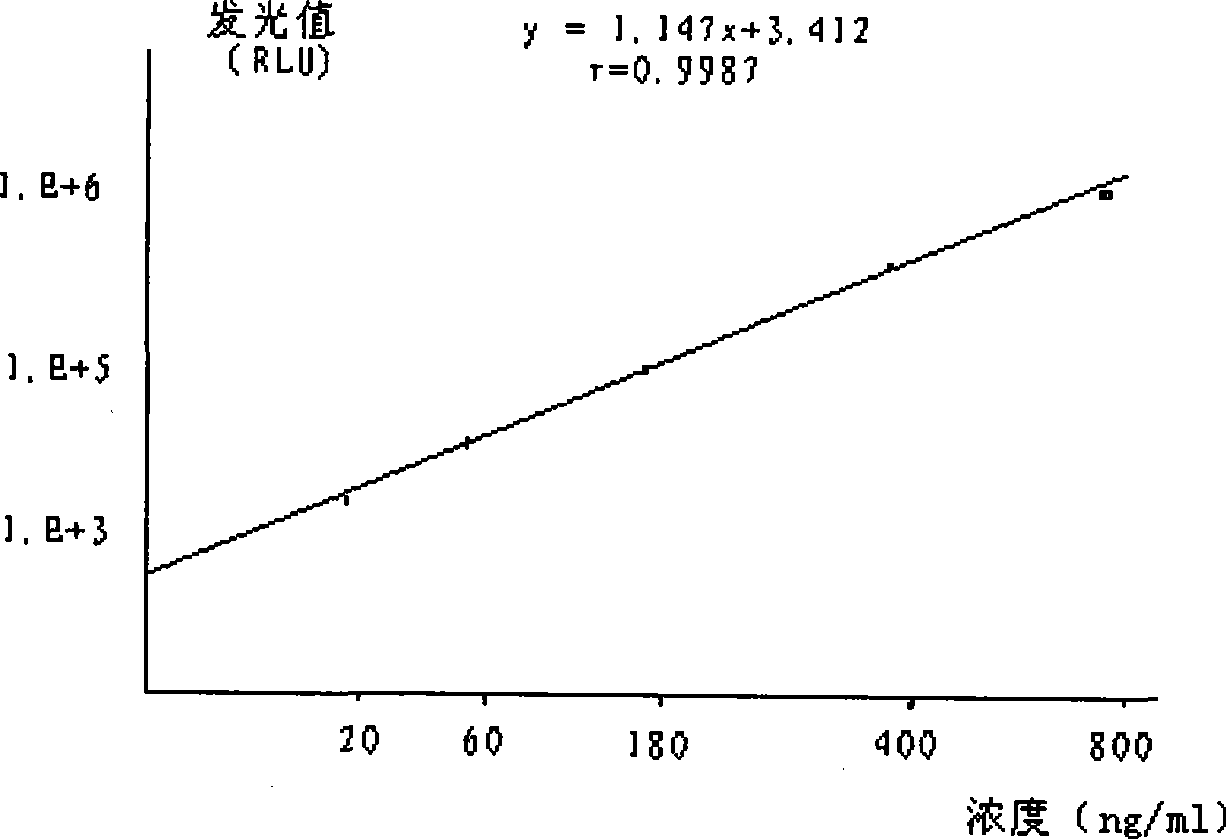
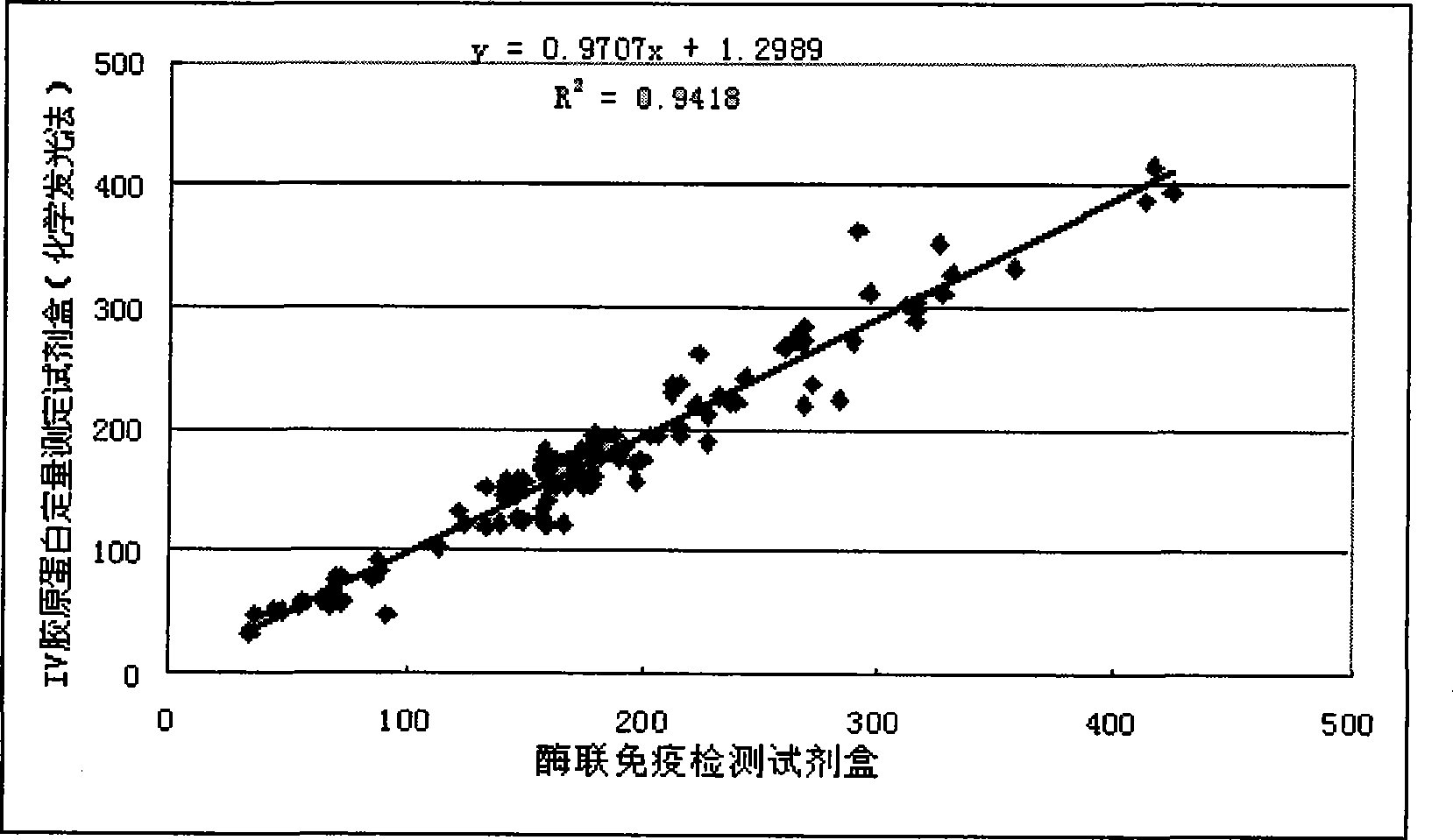
Image
Examples
Embodiment 1
[0039] Example 1 Preparation of Type IV Collagen Quantitative Assay Kit of the present invention
[0040] 1. Preparation of enzyme-labeled antibody and biotin-conjugated antibody
[0041] 1. Preparation of horseradish peroxidase-labeled type IV collagen antibody
[0042] Dissolve 4.4mg HRP in 1mL distilled water, add 0.4mL sodium periodate (50mmol / L) and stir at room temperature for 20min, dialyze through 1mmol / L sodium acetate buffer solution, pH value is 4.4, add 8mg type IV collagen monoclonal antibody, stir for 2h , and finally reduced with 200mmol / L NaBH4, dialyzed through 0.02MPB buffer solution, added an equal volume of glycerol, and stored below -20°C.
[0043] 2. Preparation of biotin-conjugated type IV collagen antibody
[0044] (1) Dissolve biotin (BNHS) in N, N-dimethylformamide (DMF) by conventional methods to make 1 mg / mL;
[0045] (2) Use 0.1mol / L NaHCO with a pH value of 9.0 3 Dilute the purified type IV collagen antibody to 1-2 mg / mL;
[0046] (3) Mix acc...
Embodiment 2~3
[0101] Embodiment 2~3 preparation IV collagen quantitative assay kit of the present invention
[0102] Except for using plastic beads and plastic tubes as carriers, the rest were prepared in the same manner as in Example 1 to prepare a type IV collagen chemiluminescence immunoassay assay kit.
Embodiment 4
[0103] Example 4 Preparation of Type IV Collagen Quantitative Assay Kit of the present invention
[0104] Except for using magnetic particles as the coating carrier, using the glutaraldehyde method to couple with avidin, the other methods were the same as in Example 1 to prepare a type IV collagen chemiluminescence immunoassay assay kit.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap