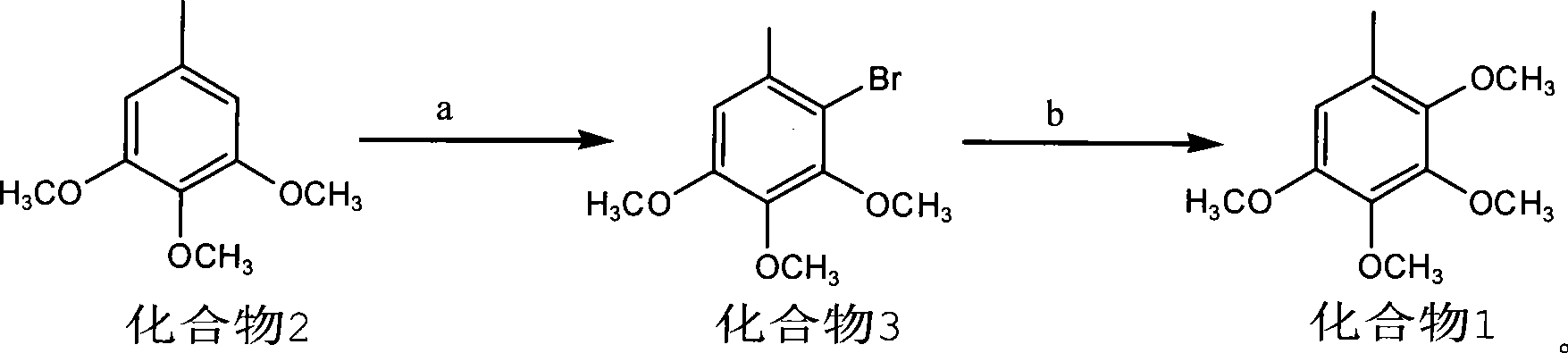
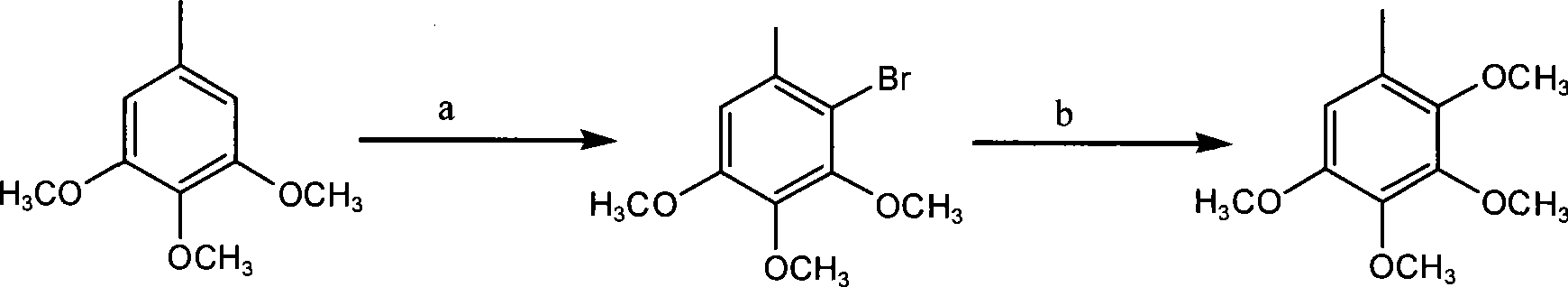
Method for preparing 2,3,4,5-tetramethoxytoluene
A technology of tetramethoxytoluene and trimethoxytoluene, applied in the field of preparation of 2,3,4,5-tetramethoxytoluene, which can solve the problem of unsynthesized routes and process research, harsh reaction conditions, and inability to apply production, etc. problem, to achieve the effect of complete environmental friendliness, reduction of reaction steps, and reduction of product production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1) Preparation of Compound 3: Weigh 18.2g (0.10mol) of raw material 3,4,5-trimethoxytoluene and place it in a 250ml three-necked flask, add 100ml of acetic acid and stir to dissolve, add NaBr11.0g (0.11mol), then Add 30% H dropwise at room temperature 2 o 2 About 50ml, react for 3-4 hours; add 150ml of water to the reaction solution, then extract with petroleum ether (80ml×2), combine the organic phases, wash once with water, dry over anhydrous sodium sulfate, filter, evaporate the petroleum ether under reduced pressure to obtain the product It is nearly 26.0 g of colorless oil, and the yield of crude product is nearly 100%. 1 HNMR: δ 6.58(s, 1H), δ 3.86(s, 3H), δ 3.83(s, 3H), δ 3.81(s, 3H), δ 2.33(s, 3H). MS(z / e): 260 .
[0019] The crude compound 3 was directly used in the next reaction without purification by distillation.
[0020] 2) Preparation of Compound 1: Weigh 26.0g (0.10mol) of Compound 3 in a 500ml dry three-necked flask, N 2 Add 6.2g (0.20mol) of sodiu...
Embodiment 2
[0022] Preparation of Compound 3: Weigh 18.2g (0.10mol) of raw material 3,4,5-trimethoxytoluene and place it in a 250ml three-necked flask, add a mixed solvent of 10ml acetic acid and 90ml tetrahydrofuran and stir to dissolve, add NaBr11.0g (0.11 mol), and then dropwise added 30% H at room temperature 2 o 2 About 40ml, react for 3-4 hours; add 150ml of water to the reaction solution, then extract with petroleum ether (80ml×2), combine the organic phases, wash once with water, dry over anhydrous sodium sulfate, filter, evaporate the petroleum ether under reduced pressure to obtain the product It is a colorless oil, and the yield is basically the same as in the above examples.
[0023] The preparation of compound 1: take by weighing 26.0g (0.10mol) compound 3 and place in 500ml dry three-necked flask, N 2 Add 6.2g (0.20mol) of sodium methoxide and 10ml of methanol under protection, stir well, then add 1.0g of CuBr and 5.0ml of DMF, heat the reaction mixture to 110-120°C for 3-...
Embodiment 3
[0025] Preparation of Compound 3: Weigh 18.2g (0.10mol) of raw material 3,4,5-trimethoxytoluene and place it in a 250ml three-necked flask, add a mixed solvent of 10ml formic acid and 90ml methanol and stir to dissolve, add NaBr 11.0g (0.11 mol), then slowly add 50ml of an aqueous solution containing 30.0g (0.11mol) of potassium persulfate at 45°C, and react for 7-8 hours; add 150ml of water to the reaction solution, then extract with petroleum ether (80ml×2), and combine The organic phase was washed once with water, dried over anhydrous sodium sulfate, filtered, and the petroleum ether was distilled off under reduced pressure to obtain the product as a colorless oil. Yield is basically the same as above embodiment.
[0026] The preparation of compound 1 is the same as the above-mentioned examples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com