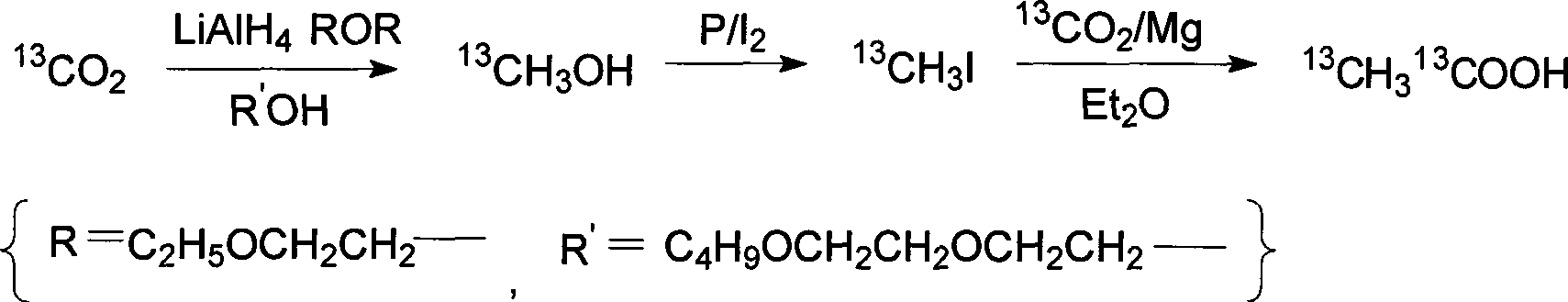Double label <13>C2-acetic acid preparation method
A double labeling and acetic acid technology, which is applied in the preparation of carboxylate, organic compound, organic chemical methods, etc., can solve the problems of complex operation, high equipment requirements, high cost, etc., and achieve high chemical purity, simple equipment, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] a double marker 13 C 2 -The preparation method of acetic acid, this method comprises the following steps:
[0035] (1) 13 CH 3 Synthesis of OH
[0036] Concentrated sulfuric acid was added dropwise to Ba 13 CO 3 (59.1 g, Atom% = 98.25%), resulting in 13 CO 2 Introduce into electromagnetic stirrer containing LiAlH within 3-4 hr 4 (5.7 grams), diethylene glycol diethyl ether (1L) in the reaction vessel, reaction temperature 20 ℃; Diethylene glycol monobutyl ether (540 grams) was added dropwise in the reaction mixture in 2-3 hours, reaction temperature 80 ℃, the mixture is distilled under reduced pressure, and the product is evaporated into a liquid nitrogen cooling receiver, and the distillation is repeated several times to obtain anhydrous high-purity 13 CH 3 OH (3.2 g).
[0037] (2) 13 CH 3 Synthesis of I
[0038] Get from step (1) 13 CH 3 OH (3.2 g) and red phosphorus (3.2 g) were mixed, reacted in a water bath at 0 ° C, added elemental iodine (12.7 g) ...
Embodiment 2
[0042] a double marker 13 C 2 -The preparation method of acetic acid, this method comprises the following steps:
[0043] (1) 13 CH 3 Synthesis of OH
[0044] Concentrated sulfuric acid was added dropwise to Ba 13 CO 3 (59.1 g, Atom% = 98.25%), resulting in 13 CO 2 Introduce into electromagnetic stirrer containing LiAlH within 3-4 hr 4 (17.1 grams), diethylene glycol diethyl ether (1L) in the reaction vessel, the reaction temperature -20 ° C; diethylene glycol monobutyl ether (245 grams) was added dropwise to the reaction mixture within 2-3 hours, the reaction temperature was 60 ℃, the mixture is distilled under reduced pressure, and the product is evaporated into a liquid nitrogen cooling receiver, and the distillation is repeated several times to obtain anhydrous high-purity 13 CH 3 OH (3.9 g).
[0045] (2) 13 CH 3 Synthesis of I
[0046] Take step (1) to get 13 CH 3 OH (3.2 grams) and red phosphorus (3.2 grams) were mixed, reacted in a water bath at -10 ° C,...
Embodiment 3
[0050] a double marker 13 C 2 -The preparation method of acetic acid, this method comprises the following steps:
[0051] (1) 13 CH 3 Synthesis of OH
[0052] Concentrated sulfuric acid was added dropwise to Ba 13 CO 3 (59.1 g, Atom% = 98.25%), resulting in 13 CO 2 Introduce into electromagnetic stirrer containing LiAlH within 3-4 hr 4 (57 grams), diglyme (1L) in the reaction vessel, reaction temperature -5 ℃; tetrahydrofurfuryl alcohol (178 grams) was added dropwise in the reaction mixture in 2-3 hours, reaction temperature 120 ℃, The mixture is distilled under reduced pressure, and the product is evaporated into a liquid nitrogen cooling receiver, and the distillation is repeated several times to obtain anhydrous high-purity 13 CH 3 OH (4.1 g).
[0053] (2) 13 CH 3 Synthesis of I
[0054] Take step (1) to get 13 CH 3 OH (3.2 g) and red phosphorus (3.2 g) were mixed, reacted in a water bath at 30 ° C, added elemental iodine (12.7 g) in batches, stopped after 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com