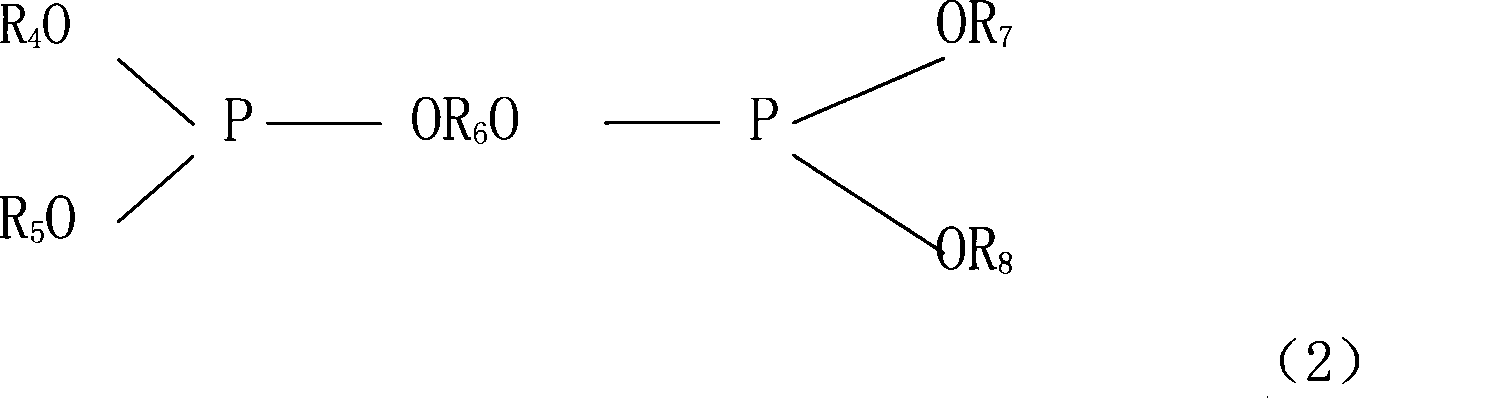
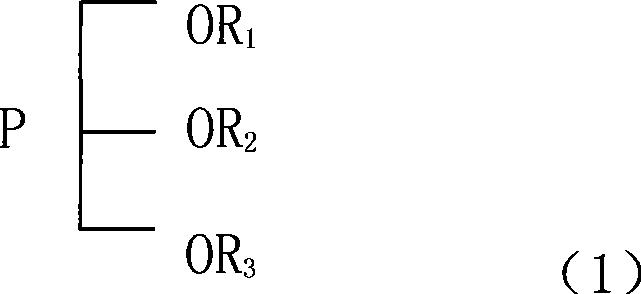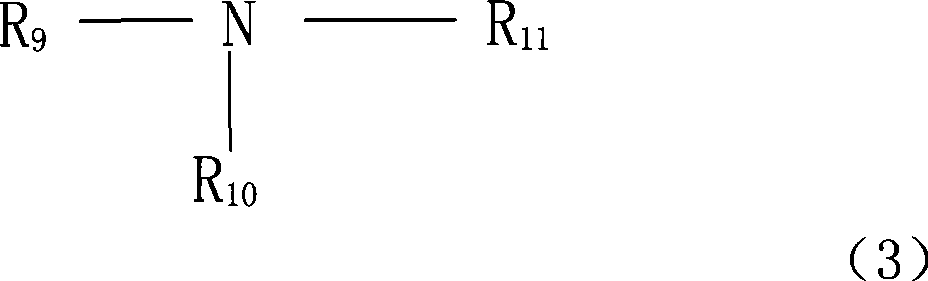Stabilizer for non-water electrolysis and non-water electrolysis containing the stabilizer
A non-aqueous electrolyte and stabilizer technology, applied in the field of electrolyte, can solve problems such as content increase, discoloration of non-aqueous electrolyte, poor thermal stability, etc., to prevent oxidative discoloration, stabilize non-aqueous electrolyte, and prevent excessive acidity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of stabilizer: In a glove box with dry gas (such as air, nitrogen or argon, its moisture content is less than 20PPM), accurately weigh 4.5 grams of trimethyl phosphite and 0.5 grams of 1,1, 3,3-tetramethyl-1,3-diphenyldisilazane, and then the above 4.5 grams of trimethyl phosphite and 0.5 grams of 1,1,3,3-tetramethyl-1,3-di Add phenyldisilazane (the order of adding the above two substances is not limited) to the first dry container (conical flask can be used), and stir or vibrate to mix the above two substances evenly to obtain 5 g stabilizer. The resulting stabilizer consisted of 90% by weight of trimethyl phosphite and 10% by weight of 1,1,3,3-tetramethyl-1,3-diphenyldisilazane.
[0032] Preparation of non-aqueous electrolyte: In a glove box with a dry gas (such as air, nitrogen or argon, the moisture content of which is less than 20PPM), accurately weigh 8 grams of lithium hexafluorophosphate, 40 grams of dimethyl carbonate, and 30 grams of dimethyl carb...
Embodiment 2
[0037] The steps of the method for preparing the stabilizer and the non-aqueous electrolyte in this example are the same as those in Example 1, but the raw materials used and their proportions are different.
[0038] Preparation of a stabilizer: The raw materials used are 2.1 grams of dibutyl phosphite and 0.9 grams of heptamethyldisilazane to obtain 3 grams of a stabilizer. The resulting stabilizer consisted of 70% by weight of dibutyl phosphite and 30% by weight of heptamethyldisilazane.
[0039] Preparation of non-aqueous electrolyte: raw materials used are 20 grams of ethylene carbonate, 50 grams of ethyl acetate, 17 grams of ethyl methyl carbonate, 10 grams of lithium hexafluorophosphate, and 3 grams of the above-mentioned stabilizer. Contain in the non-aqueous electrolytic solution that makes: the organic solvent (ethylene carbonate, ethyl acetate and ethyl methyl carbonate) of 87% (weight), the lithium salt (lithium hexafluorophosphate) of 10% (weight) and 3% (weight) ...
Embodiment 3
[0044] The steps of the method for preparing the stabilizer and the non-aqueous electrolyte in this example are the same as those in Example 1, but the raw materials used and their proportions are different.
[0045] Preparation of stabilizer: The raw materials used are 0.4 g of triethyl phosphite and 0.6 g of N,N-dimethyltrimethylsilazane to prepare 1 g of stabilizer. The prepared stabilizer consisted of 40% by weight of triethyl phosphite and 60% by weight of N,N-dimethyltrimethylsilazane.
[0046] Preparation of non-aqueous electrolyte solution: raw materials used are 30 grams of ethylene carbonate, 30 grams of dimethyl carbonate, 25 grams of N,N-dimethylformamide, 14 grams of lithium hexafluorophosphate, and 1 gram of the above-mentioned stabilizer. Contain in the nonaqueous electrolytic solution that makes: the lithium salt (lithium hexafluorophosphate) of 85% (weight) organic solvent (ethylene carbonate, dimethyl carbonate and N, N-dimethylformamide), 14% (weight) and 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com