Double-cyclitol medicine composition containing surfactant and preparation method thereof
A technology of surfactants and co-surfactants, which is applied in the field of bicyclic alcohol-containing surfactant-containing pharmaceutical compositions and its preparations, can solve the problems of no precipitation of bicyclic alcohol solids, improve bioavailability, and improve clinical efficacy. Curative effect, enhanced absorption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] [Prescription Composition]
[0073] Bicyclol 40mg
[0074] LABRAFIL M 1944CS 200mg
[0075] Cremophor RH40 300mg
[0076] Tween-80 300mg
[0077] PEG400 200mg
[0078] Propyl gallate (antioxidant) 0.01%
[0079] [Preparation method] According to the prescription, weigh the excipients, heat to 60-70°C and mix evenly; add the prescribed amount of bicyclic alcohol, stir and dissolve at 60-70°C to obtain a homogeneous solution.
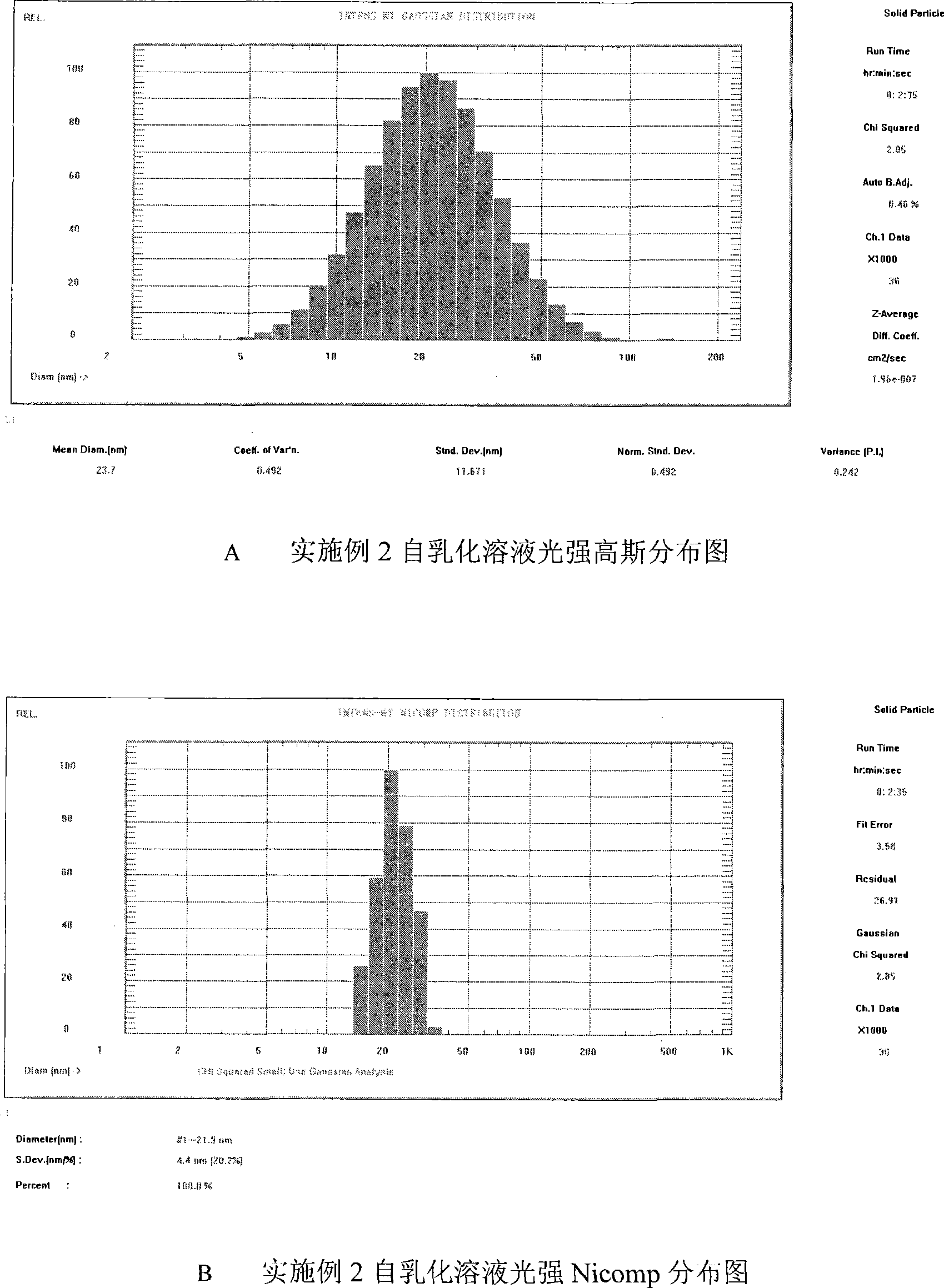
[0080] [Emulsification performance inspection] Method: Take the liquid medicine, add about 20 times of water at 37°C, shake or stir, observe the emulsification and dispersion of the liquid medicine in water; and measure the particle size distribution with a Nicomp laser particle size analyzer.
[0081] Results: the medicinal composition is light blue transparent microemulsion after adding water, and the particle size distribution range is 20nm-50nm. The particle size distribution chart is attached figure 1 .
[0082] [Bioavailability experime...
Embodiment 2
[0087] [Prescription Composition]
[0088] Bicyclol 40mg
[0089] LABRAFIL M 1944CS 200mg
[0090] Cremophor RH40 400mg
[0091] Tween-80 200mg
[0092] PEG400 100mg
[0093] Propyl gallate (antioxidant) 0.01%
[0094] [Preparation method] with embodiment 1.
[0095] [Emulsification performance inspection] with embodiment 1.
[0096] Results: the medicinal composition is light blue transparent microemulsion after adding water, and the particle size distribution range is 20nm-50nm. The particle size distribution chart is attached image 3 .
Embodiment 3
[0098] [Prescription Composition]
[0099] Bicyclol 40mg
[0100] Tween-80 600mg
[0101] PEG400 200mg
[0102] [Preparation method] with embodiment 1.
[0103] [Emulsification performance inspection] with embodiment 1.
[0104] Result: the pharmaceutical composition becomes a transparent solution after adding water.
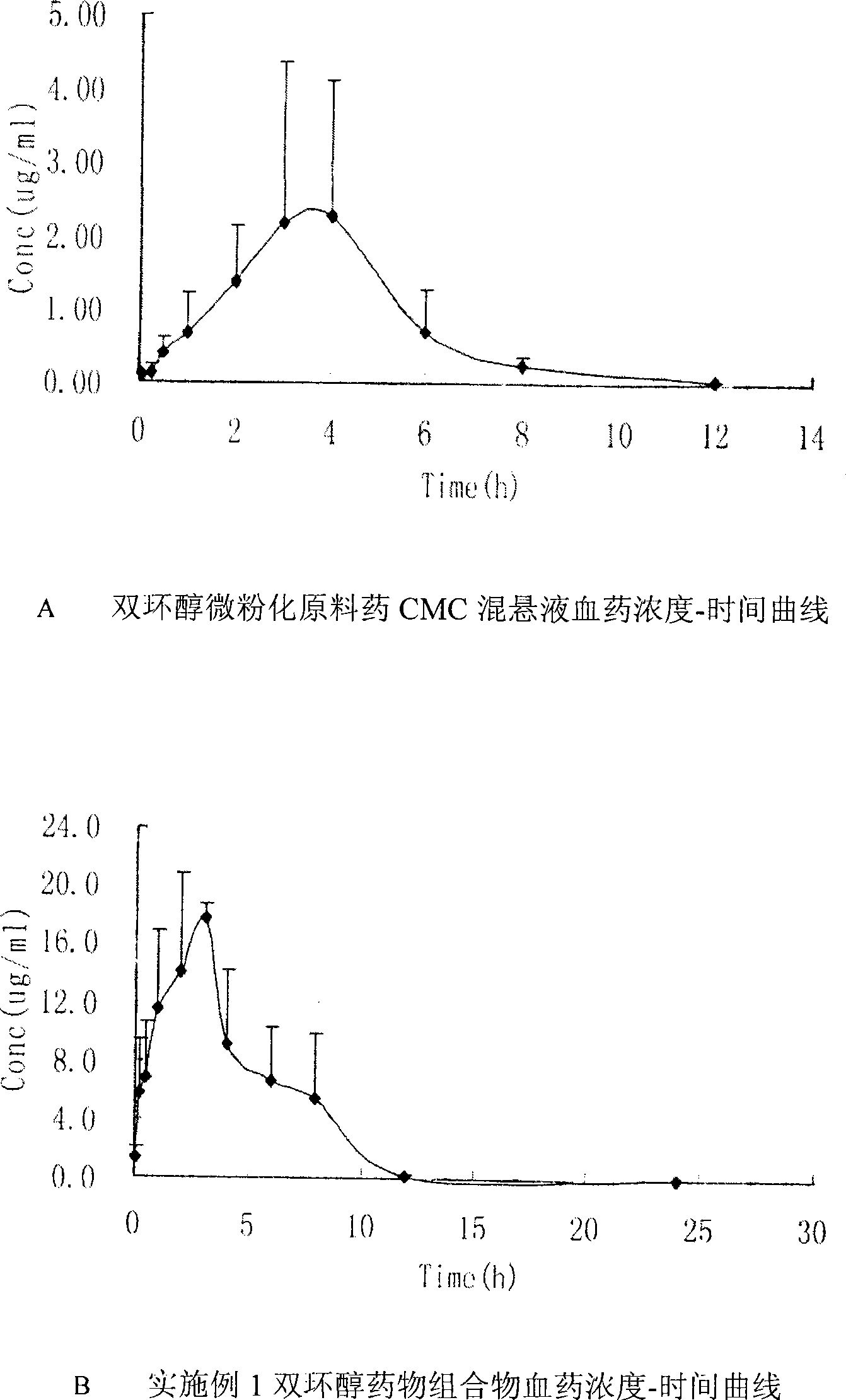
[0105] [Bioavailability experiment] Test purpose: To compare the in vivo pharmacokinetic characteristics of rats after oral administration of the bicyclol pharmaceutical composition in Example 3 and bicyclol micronized crude drug CMC suspension.
[0106] Method: Single intragastric administration of SD rats ① Bicyclol pharmaceutical composition in Example 3 ② Bicyclol micronized raw drug CMC suspension 100mg / kg, 5min, 15min, 30min, 1h, 2h, 3h after administration , 4h, 6h, 8h, 12h, 24 each time point from the canthus vein from the blood, HPLC blood sample determination, draw the blood concentration - time curve, calculate the bioavailability.
[0107] Resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com