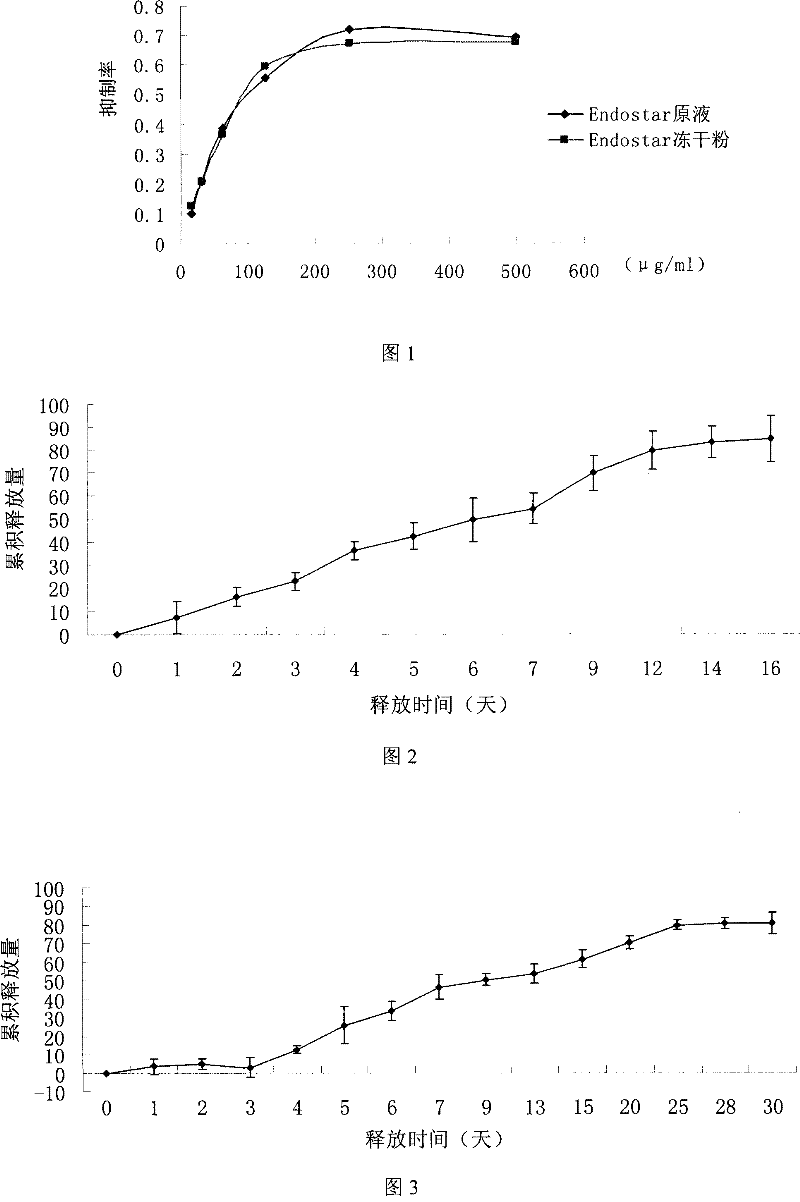
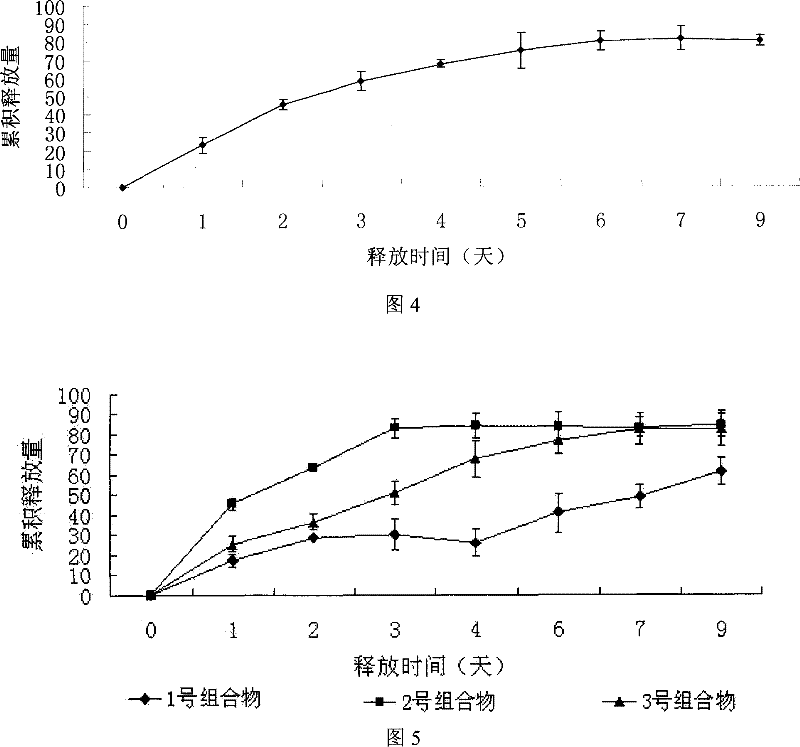
Recombined human blood vessel endothelial inhibin sustained-release injection composition
A technology of vascular endothelium and inhibin, which is applied in the field of medicine, can solve the problems of loss of protein activity, complex and difficult industrialization of microsphere preparations, etc., and achieve the effects of maintaining protein activity, mild preparation conditions, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Dissolve 650mg of PLGA (Mw=15000, polylactic acid:glycolic acid=50:50, provided by Shandong Institute of Medical Devices) in 1.16ml of N-methyl-2-pyrrolidone, shake and dissolve in a water bath at 37°C for 24 hours. Accurately weigh 0.6g of the polymer solution in a 5ml PE tube, add Endostar 0.032g freeze-dried powder (wherein the excipient is sucrose, and the mass ratio of the excipient to recombinant human endostatin is 0.5:1), use once Mix by syringe aspiration approximately 100 times. A composition was obtained, wherein the polymer accounted for 33.9% by weight of the composition, the solvent accounted for 62.7% of the composition, and Endostar was 10% by weight of the polymer. Take an appropriate amount of the mixed composition, add it to the bottom of a 5ml PE tube, then add 1ml of phosphate buffer (0.05mol / L, pH7.4), PLGA is solidified to form a sustained release system, and the sealed PE tube is placed at a constant temperature of 37°C in a water bath shaker. ...
Embodiment 2
[0031] Add 0.419ml N-methyl-2-pyrrolidone and 0.376ml glyceryl triacetate to 650mg PLGA (Mw=13000, polylactic acid:glycolic acid=65:35, provided by Durect, USA), and dissolve in a water bath at 37°C for 48 hours . Accurately weigh 0.7g of the polymer solution in a 5ml PE tube, add Endostar 0.060g freeze-dried powder (wherein the excipient is mannitol, and the mass ratio of the excipient to recombinant human endostatin is 3: 1), use Mix approximately 100 times with a single-use syringe. A composition was obtained, wherein the polymer accounted for 42.0 wt% of the composition, the solvent accounted for 55.9% of the composition, and Endostar was 5 wt% of the polymer. Take an appropriate amount of the mixed composition, add it to the bottom of a 5ml PE tube, then add 1ml of phosphate buffer (0.05mol / L, pH7.4), PLGA is solidified to form a sustained release system, and the sealed PE tube is placed at a constant temperature of 37°C in a water bath shaker. Take 0.1ml of the releas...
Embodiment 3
[0033] Add 1.195ml of methyl benzoate to 650mg of PLGA (Mw=15000, polylactic acid:glycolic acid=50:50), and dissolve in a water bath at 37°C for 48 hours. Accurately weigh 0.6g of the polymer solution into a 5ml PE tube, add 0.195g of Endostar spray-dried powder, and use a disposable syringe to pump and mix about 100 times. A composition was obtained, wherein the polymer accounted for 30.3% by weight of the composition, the solvent accounted for 60.6% of the composition, and Endostar was 30% by weight of the polymer. Take an appropriate amount of the mixed composition, add it to the bottom of a 5ml PE tube, then add 1ml of phosphate buffer (0.05mol / L, pH7.4), PLGA is solidified to form a sustained release system, and the sealed PE tube is placed at a constant temperature of 37°C in a water bath shaker. Take 0.1ml of the release solution in the PE tube of each sampling point, and add the same volume of fresh phosphate buffer at the same time. The content of Endostar in the sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com