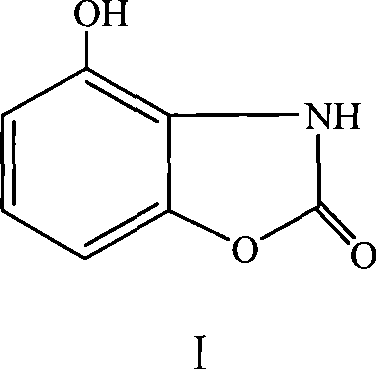
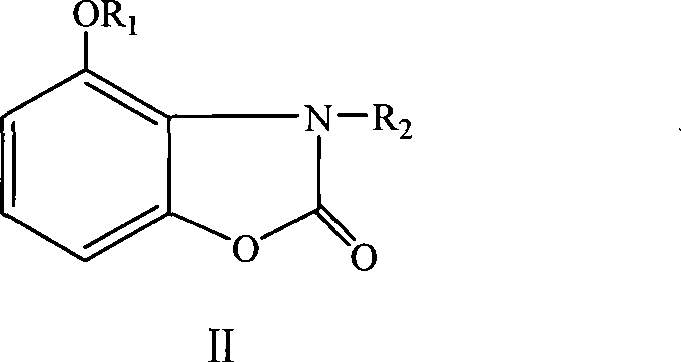
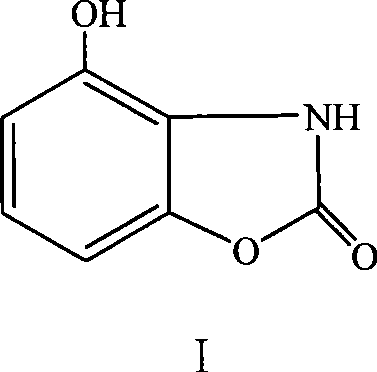
Method for preparing acanthus alkaloid A with anti inflammatory and analgesic activities and derivatives thereof
An anti-inflammatory, analgesic, and rat-like technology, applied in the directions of organic active ingredients, medical preparations containing active ingredients, anti-inflammatory agents, etc., can solve the problems of high price of carbonyl diimidazole, environmental pollution, etc. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The method for preparing bougainvillea alkaloid A:
[0029] Mix 16.16g (0.10mol) of 2-aminoresorcinol hydrochloride and 30.04g (0.60mol) of urea and put it into a 250mL three-necked flask. For about 1h, continue to heat up to 150°C for about 1h. Finally, heat up to 190°C until no NH3 escapes, which lasts for about 6h. Heating was stopped, cooled to obtain a solid, and the solid was washed several times with cold water to obtain a crude product. Heat the crude product in n-butanol to reflux, filter to remove insoluble matter, concentrate the filtrate to dryness, add 80% ethanol to dissolve the obtained solid, reflux, cool slightly, add an appropriate amount of activated carbon, boil for several minutes, filter while hot, and concentrate to an appropriate amount. Cool to precipitate crystals, filter with suction, and dry at 80°C to 100°C to constant weight to obtain white bougainvillea alkaloid A. Yield 60%. mp: 293°C to 294°C.
Embodiment 2
[0031] The method for preparing 4-butyryloxybenzoxazol-2-one:
[0032] Add 4.53g (0.03mol) of bougainvillea alkaloid A and 7.58g (0.075mol) of triethylamine into the three-necked flask, dissolve it in acetone, and dilute with acetone under stirring in an ice bath at 5°C to 10°C. 4.79g (0.045mol) of butyryl chloride, after about 30 minutes of dripping, reacted in an ice-water bath at 0°C to 5°C for 2h, transferred to a water bath at 40°C to 45°C for 3h, raised the temperature to reflux, and tracked and detected the raw material point by TLC Disappeared, stopped the reaction, cooled to room temperature. Neutralize to neutral with 0.1mol / L HCl, filter, recover acetone to obtain a light yellow oil, stand at room temperature, precipitate a white needle-like solid, filter, wash the solid with distilled water, and recrystallize with 80% ethanol to obtain a white Acicular 4-butyryloxybenzoxazol-2-one. Yield 60%. mp: 115°C to 117°C.
Embodiment 3
[0034] The method for preparing 3-methyl-4-methoxybenzoxazol-2-one:
[0035]Add 3.02 g (0.02 mol) of bougainvillea alkaloid A into the three-necked flask, add acetone to dissolve, then add 9.20 mL (0.04 mol) of 15% NaOH solution dropwise in an ice bath at 5°C to 10°C while stirring. After a large amount of white precipitate appeared, it was transferred to a water bath at 40°C, and methyl iodide diluted with acetone was added dropwise under stirring, kept for 3 hours, and then heated to reflux. The disappearance of the raw material point was detected by TLC tracking, and the reaction was stopped and cooled to room temperature. Neutralize to neutral with 0.1mol / L HCl, filter, recover acetone to obtain a solid, and wash with distilled water. The solid was dissolved in 50ml of chloroform, and the insoluble matter was removed. The filtrate was collected and evaporated to dryness in chloroform to obtain a crude product. Dissolve the crude product in an appropriate amount of 80% eth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com