Organic material and use thereof in organic EL device
An organic material and organic technology, applied in the field of organic electroluminescent display, can solve the problems of reducing the cost of OLED, increasing the complexity and disadvantages of device manufacturing process, and achieving the effect of high electron mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
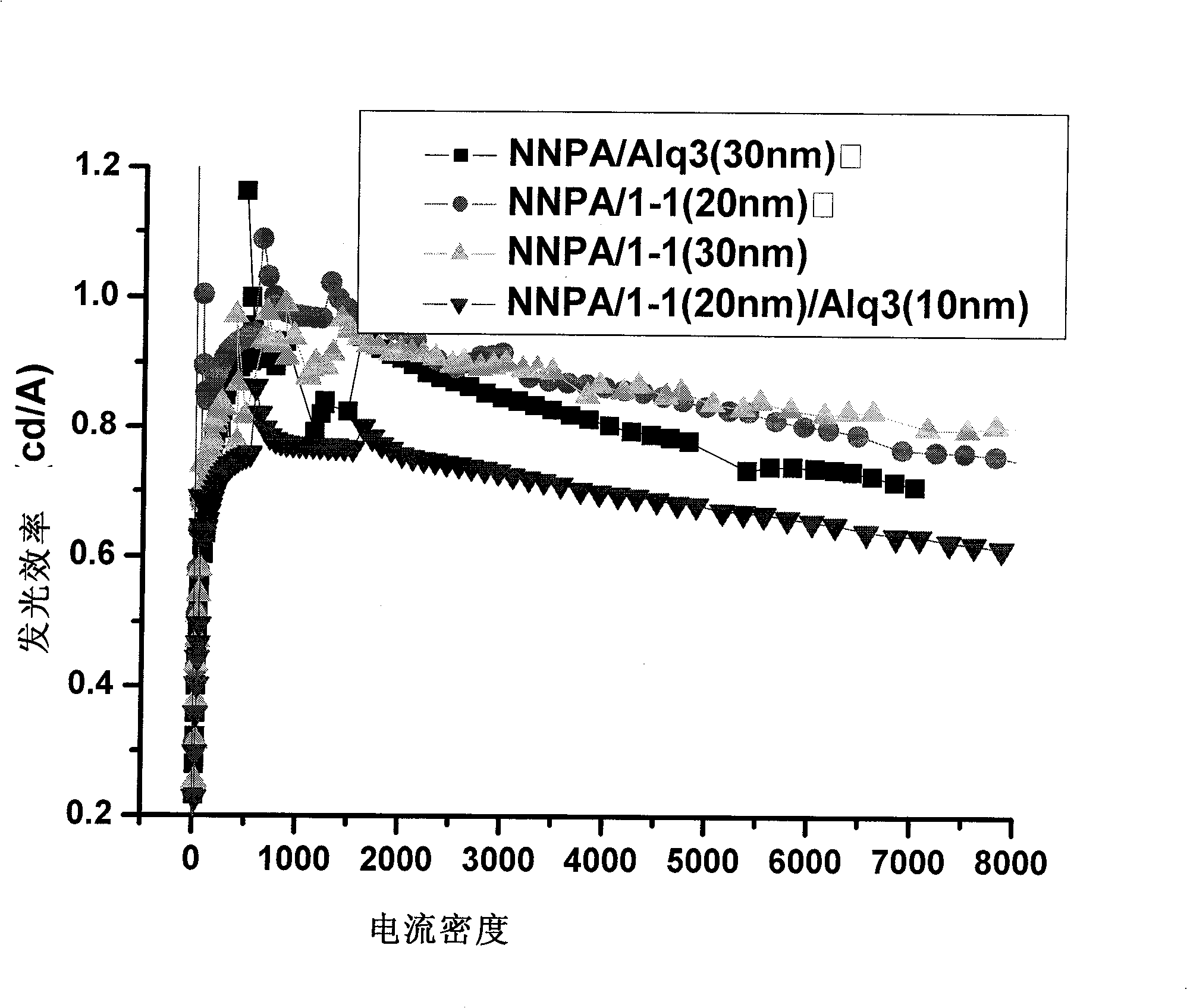
Image
Examples
Embodiment 1
[0064] The synthesis of embodiment 1 compound 1-1
[0065]
[0066] Under the protection of Ar gas, add 3.42g of 3-(4-bromophenyl)pyridine (molecular weight 234, 0.01385mol), 50ml of THF into a 500mL three-necked flask, cool to -78°C, add 5.35ml (concentration 2.8 M, 0.014mol) of BuLi (dissolved in 20ml of THF), was added in 30min, the solution was black, and stirred at -78°C for 30min.
[0067] At -78°C, 1.25 g (0.0058 mol) of anthraquinone was added as a solid, and 20 ml of THF was added. After the addition was completed, the mixture was heated to room temperature with natural stirring, and then stirred at room temperature for 2 hr. Add 200ml of water and stir. Extract with ethyl acetate, and evaporate the ethyl acetate to dryness. Add 45ml acetic acid, 4.2gKI, 4.2g sodium hypophosphite to the solid. Heating to reflux under stirring, the solution quickly changed from brown to red, then beige and precipitated. After stirring for 1 hr, filter, wash with acetic acid, wa...
Embodiment 2
[0070] The synthesis of embodiment 2 compound 1-3
[0071]
[0072] The reaction process is the same as in Example 1, except that the raw material anthraquinone is replaced by 2-phenylanthraquinone to obtain a pale yellow solid product.
[0073] Product MS (m / e): 560; Elemental analysis (C 42 h 28 N 2 ): theoretical value C: 89.97%, H: 5.03%, N: 5.00%; measured value C: 89.95%, H: 5.01%, N: 5.04%.
Embodiment 3
[0074] The synthesis of embodiment 3 compound 1-5
[0075] (1) Synthesis of anthraquinone-like structures
[0076]
[0077] Add 10g of N-phenyl-2,5-dimethylpyrrole, 2g of anhydrous AlCl to a reaction flask 3 (molecular weight: 133, 0.015 mol), stir, and keep the temperature at about 80°C, slowly add 1.06 g of phthalic anhydride (molecular weight: 148, 0.007 mol) dropwise. After the addition, keep stirring for 2 hours. After cooling down slightly, it was immediately poured into ice water containing a little HCl, and the obtained solid product was washed twice with water, separated by column chromatography to obtain 1.75 g of the target intermediate, the yield was 80.0%, and the Ms analysis was 319, which was consistent.
[0078] Add 1.75g of the above product into a dry reaction flask, then add 6g of concentrated sulfuric acid, heat to about 145°C for 1 hour with stirring, and then keep for 4 hours at about 170°C. After cooling slightly, pour it into crushed ice under s...
PUM
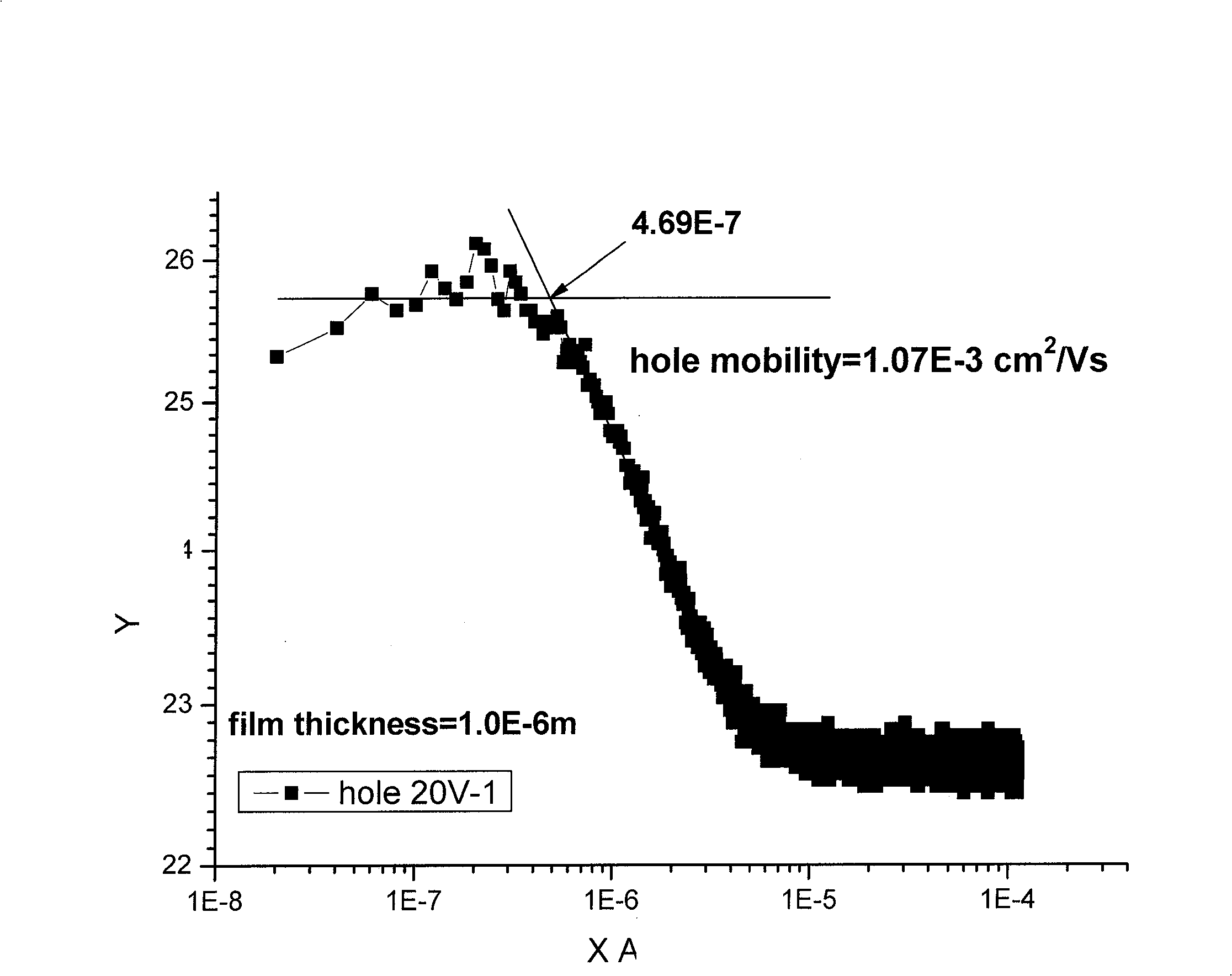
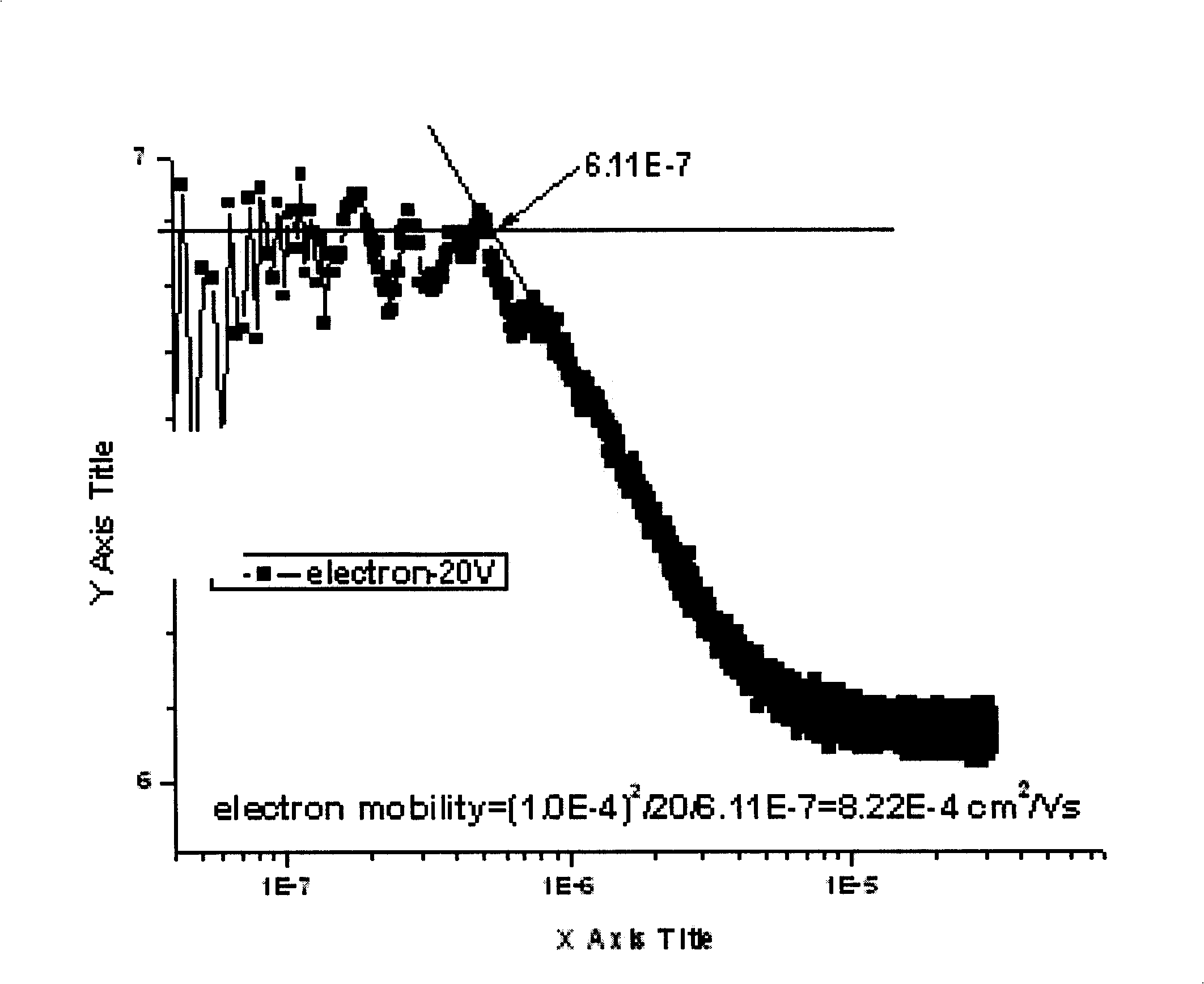
| Property | Measurement | Unit |
|---|---|---|
| Hole mobility | aaaaa | aaaaa |
| Electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com