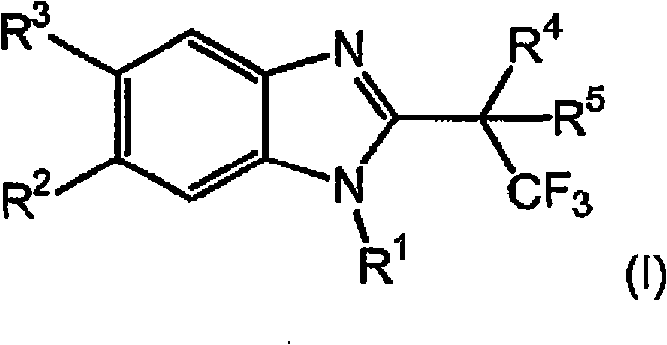
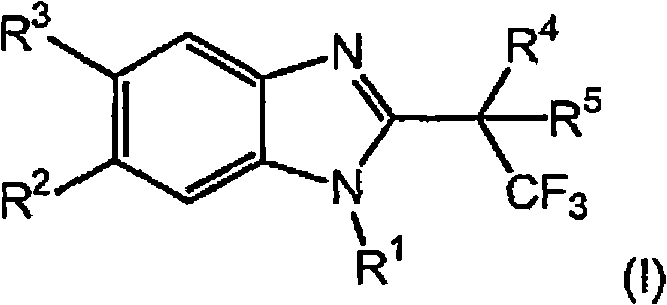
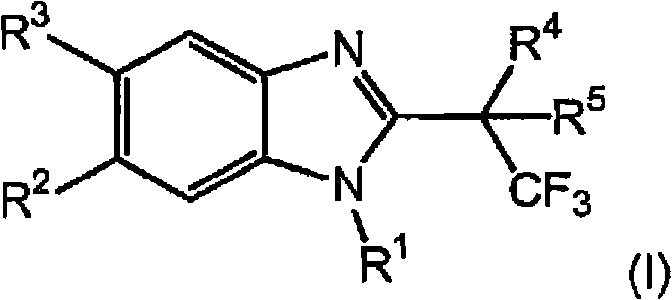
Novel 2-substituted benzimidazoles as selective androgen receptor modulators (SARMs)
A substituent, selected technology, applied in the field of application in disorders and conditions, can solve the problem of harm, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0164] 1-(5,6-dichloro-1H-benzimidazol-2-yl)-2,2,2-trifluoro-ethanol
[0165]
[0166] Into a 1-L 4-neck flask equipped with a thermocouple controller, overhead mechanical stirrer, condenser, and nitrogen inlet / outlet adapter, was charged with 4,5-dichloro-1,2-phenylenediamine (71.3 g, 0.403 mol), trifluorolactic acid (87.0 g, 0.604 mol) and 4N HCl (340 mL). The reaction mixture was heated at reflux (100° C.) for 18 h. The resulting solution was cooled to room temperature, then washed with EtOAc (1 L) and H 2 O (1L) diluted. with NaHCO 3 (500 g) the solution was worked up slowly until pH 8-9. After effervescence ceased, the phases were separated and the aqueous layer was back extracted with EtOAc (3 x 1 L). with H 2 The combined organic phases were washed with O (1 L) and brine (1 L); 4 Drying, filtering and evaporation to dryness gave a crude residue. The crude residue was subjected to flash chromatography with SiO 2 (2kg) and 10% EtOAc / CH 2 Cl 2 (2L) and 20% ...
Embodiment 2
[0168] 1-(5,6-Dichloro-1H-benzimidazol-2-yl)-2,2,2-trifluoro-ethanone
[0169]
[0170] Into a 3-L 4-neck flask equipped with a thermocouple controller, overhead mechanical stirrer, addition funnel, and nitrogen inlet / outlet adapter, was charged 1-(5,6-dichloro -1H-benzimidazol-2-yl)-2,2,2-trifluoro-ethanol (91.0g, 0.32mol), 4-methoxy-TEMPO (14.3g, 0.077mol) and KBr (4g, 0.0336 mol). The brown homogeneous solution was stirred for 15 min while cooling to -10 °C. After cooling, NaOCl (670ml) was added dropwise over a period of 1 / 2h. with EtOAc (1.5L) and H 2 O (1.5 L) diluted the reaction mixture. After effervescence ceased, the phases were separated and the aqueous layer was back extracted with EtOAc (2 L). The combined organic layers were washed with brine (2 L); 2 SO 4 Drying, filtering and evaporation to dryness gave a crude residue. The crude residue was subjected to flash chromatography with SiO 2 (1 kg) and 40% EtOAc / Hexane (24 L), and the product was dried ...
Embodiment 3
[0172] 2-(5,6-Dichloro-1H-benzimidazol-2-yl)-1,1,1-trifluoro-pent-4-en-2-ol (#1)
[0173]
[0174] 1-(5,6-Dichloro-1H-benzimidazol-2-yl)-2,2,2-trifluoro-ethanone (1.41 g; 4.99 mmol), bromopropene (0.85 mL; 10.05 mmol) and indium (1.15 g; 10.05 mmol) were suspended in THF (50 mL) and 0.01 M HCl (150 mL) and stirred vigorously over 18 hours. The layers were separated and the aqueous layer was extracted with ethyl acetate (3 x 30 mL). The combined extracts were washed with brine (50 mL) and washed with Na 2 SO 4 dry. The resulting crude brown oil was subjected to column chromatography (SiO 2 ; 20% ethyl acetate / hexanes) to afford the title compound as a tan solid.
[0175] 1 H NMR (400MHz, CD 3 CN): δ7.79(s, 2H), δ5.59(m, 1H), δ5.17(d, J=17.1Hz, 1H), δ5.07(d, J=11Hz, 1H), δ3. 13(dd, J=6.8, 14.3Hz, 1H), δ2.88(dd, J=7.2, 14.3Hz, 1H)
[0176] MS vs. C 12 h 9 Cl 2 f 3 N 2 Calculated value of O: 325.11
[0177] MS measured: 325, 327 (M+H); 323, 325 (M-1).
[0178]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com