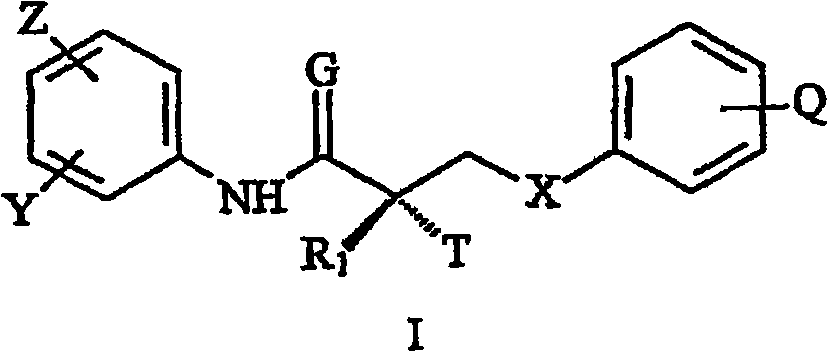
N-bridged selective androgen receptor modulators and methods of use thereof
An androgen receptor and selective technology, applied in diseases, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as easy damage, weakened physical condition, and harmful personal health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0258] The preparation of pharmaceutical compositions containing active ingredients is known in the art. Typically, such compositions are formulated as polypeptide aerosols for administration to the nasopharynx, or as injectable solutions or suspensions. However, solid forms suitable for solution in, or suspension in, liquid prior to injection can also be prepared. The formulation may also be emulsified. The active therapeutic ingredient is usually mixed with excipients which are pharmaceutically acceptable and compatible with the active ingredient. Suitable excipients are, for example, water, saline, dextrose, glycerol, ethanol, etc. or combinations thereof.
[0259] In addition, the composition can contain minor amounts of auxiliary substances, such as wetting or emulsifying agents, pH buffering agents, which enhance the effectiveness of the active ingredients.
[0260] The active ingredients can be formulated into compositions in the form of neutral pharmaceutically acce...
Embodiment 1
[0267] Synthesis of N-bridged selective androgen modulator compounds
[0268] A. Chemistry
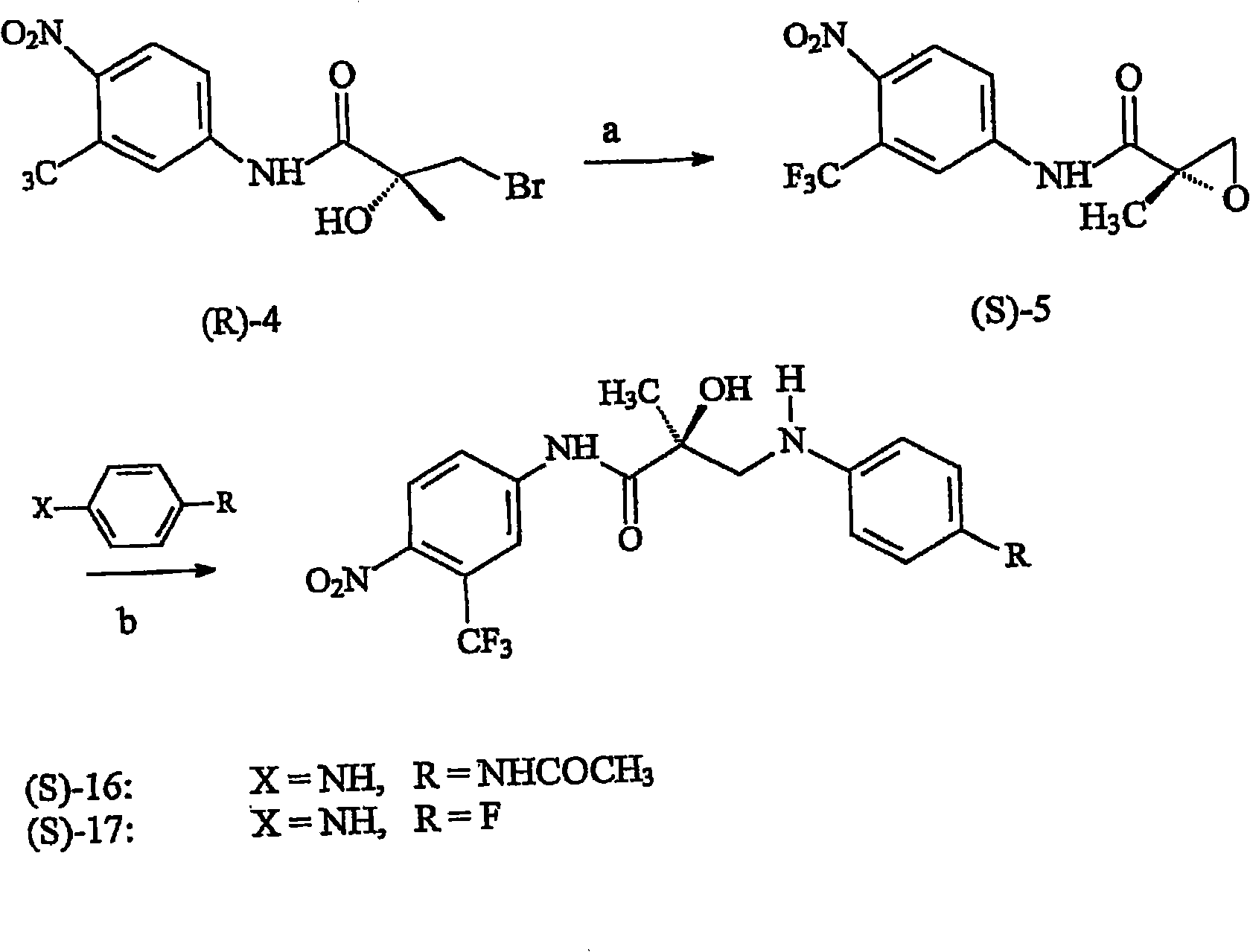
[0269] According to route 1 ( figure 1 ) to synthesize the N-bridged selective androgen modulator compound (SARM) of the present invention. First, the synthesis was carried out in two separate steps—isolating the epoxide (S)-5 and then opening the epoxide. These steps were combined into a two-step one-pot process, where after epoxide formation, the solvent was removed and the resulting residue was immediately subjected to an opening step. The first step proceeded cleanly and completely to the epoxide by TLC. The epoxide is opened with the appropriate substituted aniline in hexafluoroisopropanol. Aromatic amines are extremely non-nucleophilic; therefore the epoxide must be formed and opened in the presence of hexafluoroisopropanol, which increases the electrophilicity of the epoxide.
[0270] B. Synthesis
[0271] S-3-(4-acetylamino-phenylamino)-2-hydroxyl-2-methyl-N-(4-nitro-3-tr...
Embodiment 2
[0274] Androgen receptor binding affinity of N-bridged selective androgen modulator compounds
[0275] method
[0276] Using a competitive binding assay as previously described (Kirkovsky, L. et al., Chiralnonsteroidal affinity ligands for tbe androgen receptor. Bicalutamide analogues bearing electrophilic groups in the B aromatic ring. J. Med. Chem. 2000, 43, 581-590 ) to determine AR binding affinity. Briefly, AR binding studies were performed as follows: increasing concentrations (10 -3 nM to 10000nM) of each ligand and cytosol, and the saturating concentration 3 H-Mibobolone (MIB) (1 nm) was incubated together at 4°C for 18 hours. The incubation also contained 1000 nM triamcinolone acetonide to block the interaction of MIB with the progesterone receptor. To determine non-specific binding, a separate experiment was performed by adding 1000 nM MIB to the incubation. Separation of bound and free radioactivity was achieved at the end of the incubation by the hydroxyapatit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com