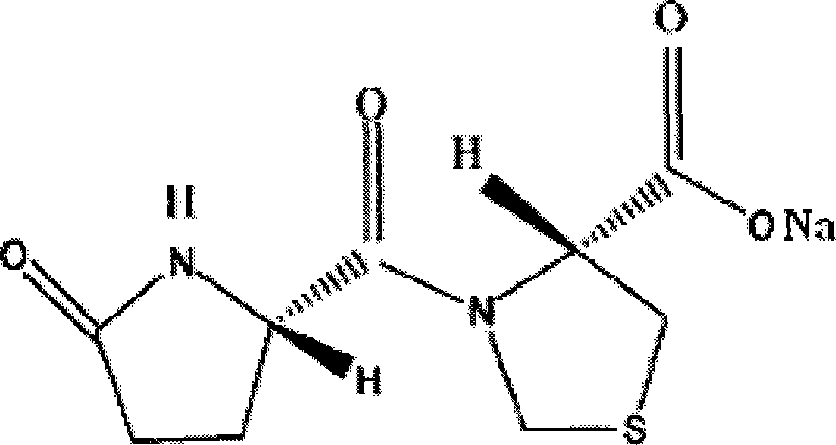
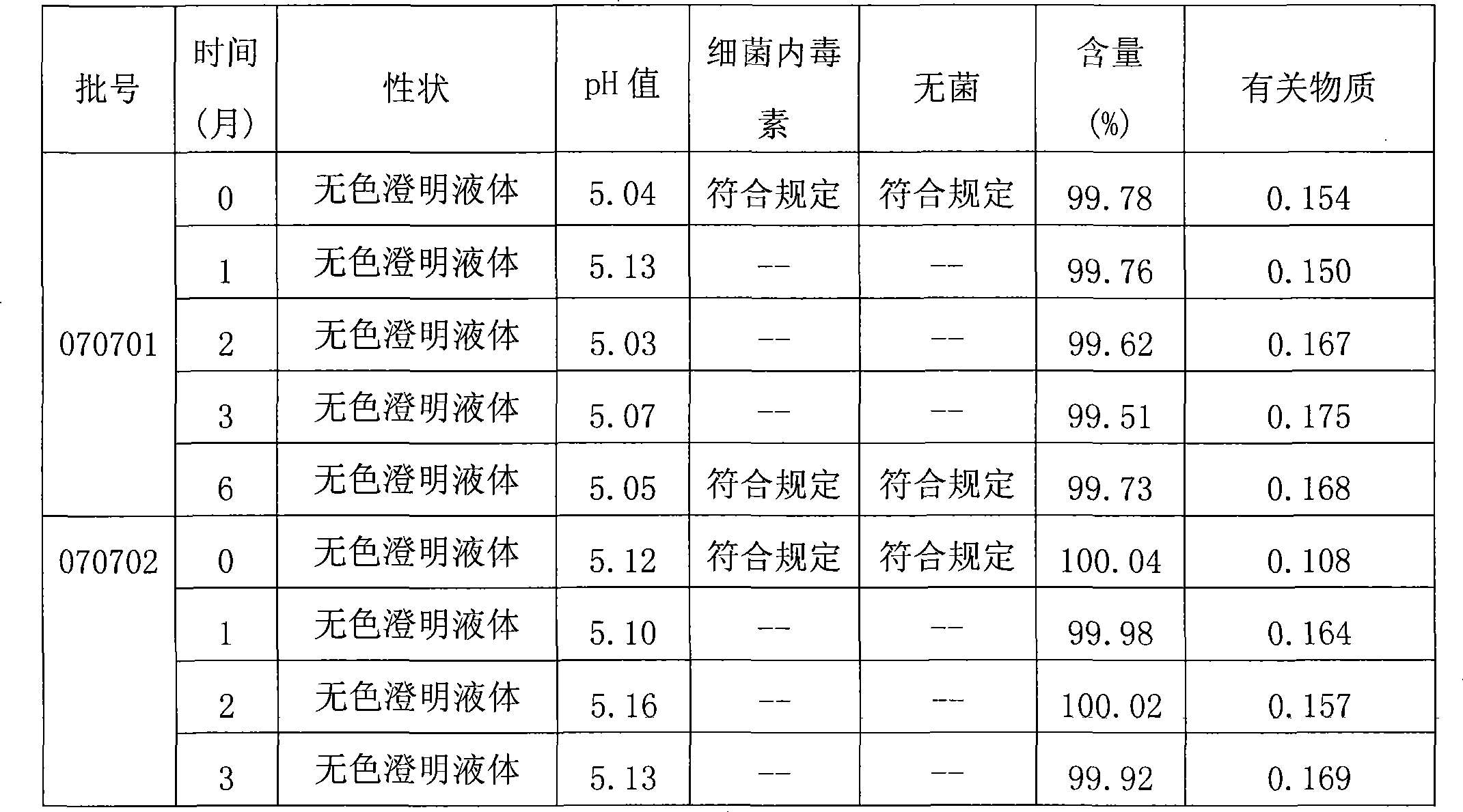
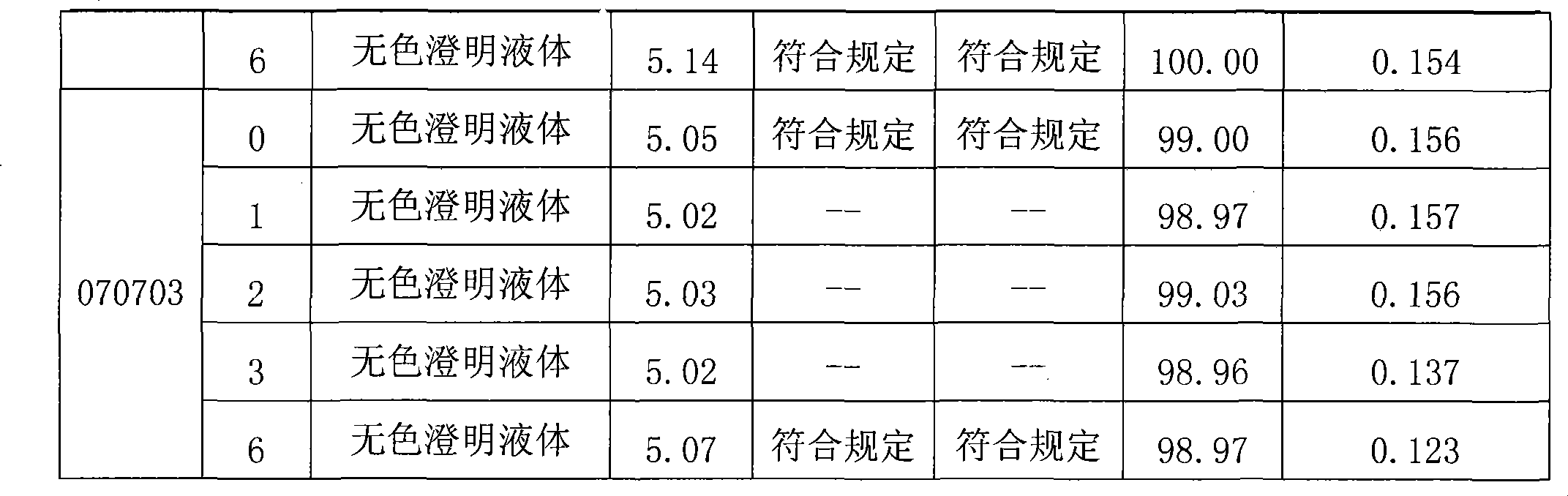
Injection containing pidotimod sodium salt
A technology of Domod sodium and injection, which is applied in the new field of medicine, can solve problems such as incomplete sterilization, product contamination, and hidden dangers of product safety, and achieve the effects of high solubility, high safety, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: injection (specification 2ml: 0.1g)
[0029] Add 244.26g of pidotimod and 40.01g of sodium hydroxide to 3500ml of water for injection, stir to dissolve completely, add 4g of charcoal for needles, and continue stirring for 20 minutes, filter to decarburize, add water for injection to 4485.2ml, Sterilize by fine filtration, subpackage, 2ml / bottle, sterilize at 121°C for 20min, and make injection solution.
Embodiment 2
[0030] Embodiment 2: injection (specification 5ml: 0.2g)
[0031] Add 244.26g of pidotimod and 40.01g of sodium hydroxide to 4000ml of water for injection, stir to dissolve completely, add 50g of sodium chloride and 4g of charcoal for needles, stir at 60°C for 20 minutes, filter for decarburization, and add injection Make up to 6106.5ml with water, sterilize by fine filtration, aliquot, 5ml / bottle, and sterilize at 121°C for 20min to make injection.
Embodiment 3
[0032] Embodiment 3: injection (specification 5ml: 0.2g)
[0033] Take 244.26g of pidotimod and 40.01g of sodium hydroxide and add it to 2000ml of ethanol, stir to dissolve completely, filter, concentrate under reduced pressure to recover ethanol, evaporate to dryness, and obtain solid powder, which is pidotimod sodium.
[0034] Add the above-mentioned pidotimod sodium into 4000ml of water for injection, stir to dissolve completely, add 4g of charcoal for needles, stir for 20 minutes, filter for decarburization, add water for injection to 6100ml, filter and sterilize, subpackage, 5ml / tube, sterilized at 121°C for 20 minutes, and made into injection solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com