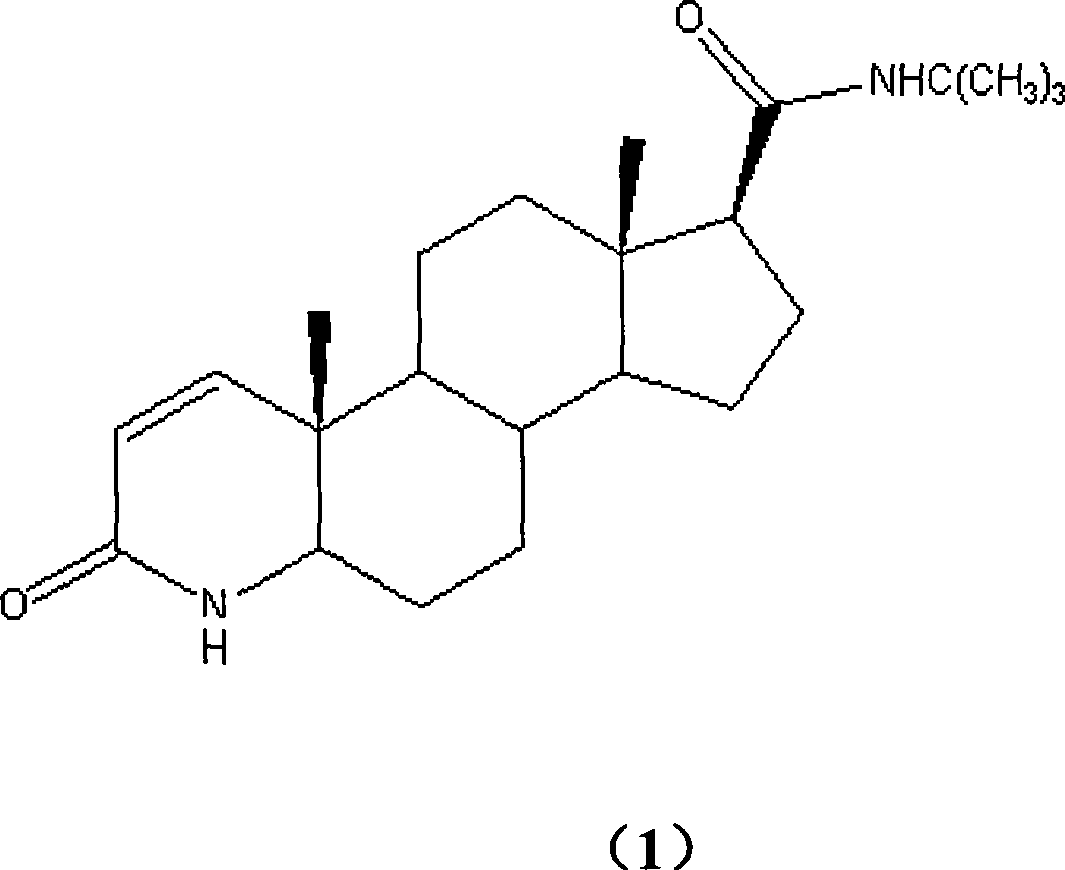
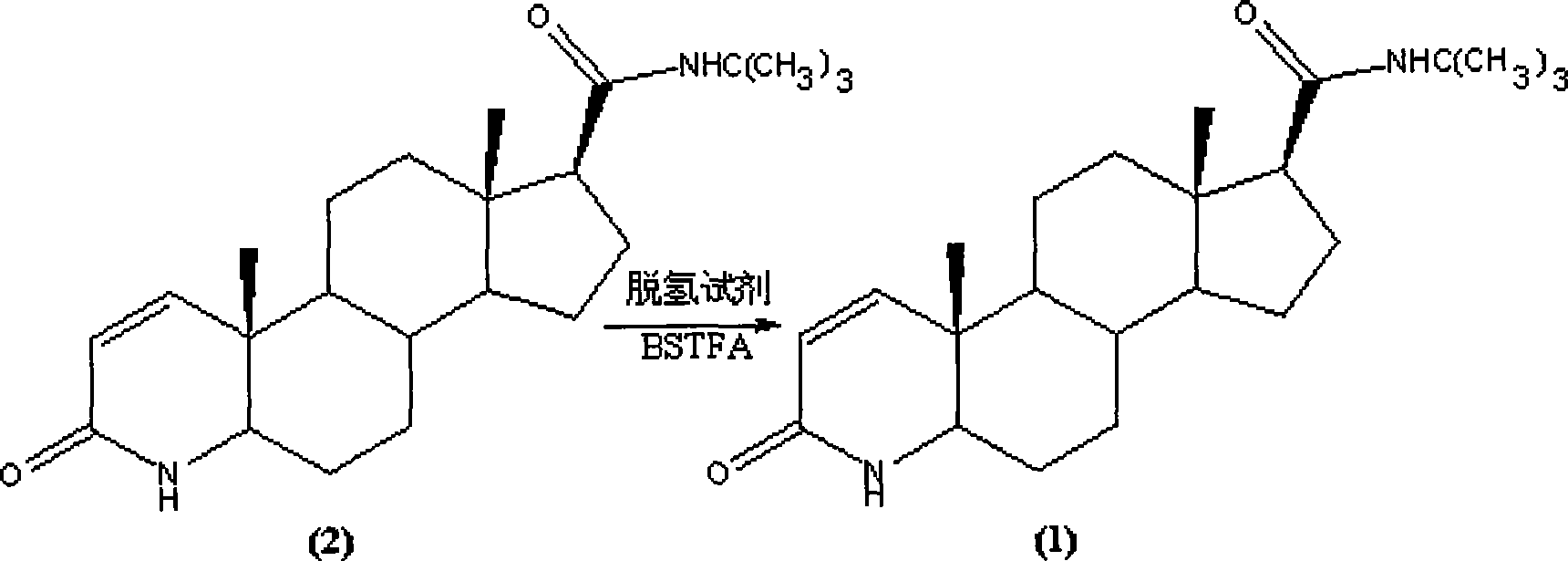
Method for synthesizing finasteride
A synthetic method and finasteride technology, applied in the field of preparation of finasteride, can solve the problems of many by-products, expensive reagents, high reflux temperature, etc., and achieve the effects of reducing the emission of "three wastes", reducing production costs, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add trifluoroacetamide (11.30g; Fw: 113.04; 100mmol), anhydrous triethylamine ( 24.29 g; Fw: 101.19; 240 mmol); then the system was stirred at room temperature for 10 min, and trimethylchlorosilane (20.64 g; Fw: 108.64; 190 mmol) was transferred to a constant pressure dropping funnel. Raise the temperature of the system to 50°C, slowly drop trimethylchlorosilane into the system, control the temperature of the system not to exceed 50°C, and complete the dropwise addition for about 2 hours. After the dropwise addition, the system continued to stir at 50° C. for 10 h. After the reaction was completed, a large amount of triethylamine hydrochloride would precipitate out of the system during the reaction.
[0029] After the reaction is complete and the system is cooled to room temperature, the system can be diluted with 50 mL of anhydrous toluene, filtered to remove triethylamine hydrochloride, and the filter cake is washed with 50 mL of anhydrous toluene, and the organic pha...
Embodiment 2
[0031] Add trifluoroacetamide (11.30g; Fw: 113.04; 100mmol), anhydrous triethylamine ( 50.60g; Fw: 101.19; 500mmol); toluene 1000mL, then the system was stirred at room temperature for 10min, and trimethylchlorosilane (54.32g; Fw: 108.64; 500mmol) was transferred to a constant pressure dropping funnel. Raise the temperature of the system to 50°C, slowly drop trimethylchlorosilane into the system, control the temperature of the system not to exceed 50°C, and complete the dropwise addition for about 2 hours. After the dropwise addition, the system continued to stir at 110° C. for 2 h. After the reaction was completed, a large amount of triethylamine hydrochloride would precipitate out of the system during the reaction.
[0032] After the reaction is complete and the system is cooled to room temperature, the system can be diluted with 50 mL of anhydrous toluene, filtered to remove triethylamine hydrochloride, and the filter cake is washed with 50 mL of anhydrous toluene, and the ...
Embodiment 3
[0034] Add trifluoroacetamide (11.30 g; Fw: 113.04; 100 mmol), 1,4-dioxo Hexacyclic 67 mL, anhydrous pyridine (7.91 g; Fw: 79.10; 100 mmol); then the system was stirred at room temperature for 10 min, and trimethylchlorosilane (10.86 g; Fw: 108.64; 100 mmol) was transferred to a constant pressure dropping funnel. Keep the system temperature at 20°C, slowly drop trimethylchlorosilane into the system, control the system temperature not to exceed 20°C, and complete the dropwise addition for about 2 hours. After the dropwise addition, the system continued to stir at 20° C. for 24 hours. After the reaction was completed, a large amount of pyridine hydrochloride was precipitated from the system during the reaction.
[0035] After the reaction is completed and the system is cooled to room temperature, the system can be diluted with 50 mL of 1,4-dioxane, filtered to remove pyridine hydrochloride, and the filter cake is washed with 50 mL of anhydrous 1,4-dioxane, and the organic phases...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com