Synthetic method of trandolapril key intermediate (2S,3aR,7as)-octahydro-1H-indole-2-carboxylic acid
A synthesis method and technology of trandolapril are applied in the field of synthesizing the angiotensin inhibitor trandolapril, which can solve the problems of low final product yield, inconvenient production and operation, low utilization rate of raw materials, etc. The effect of raw material utilization, improving raw material utilization efficiency, and reducing product cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
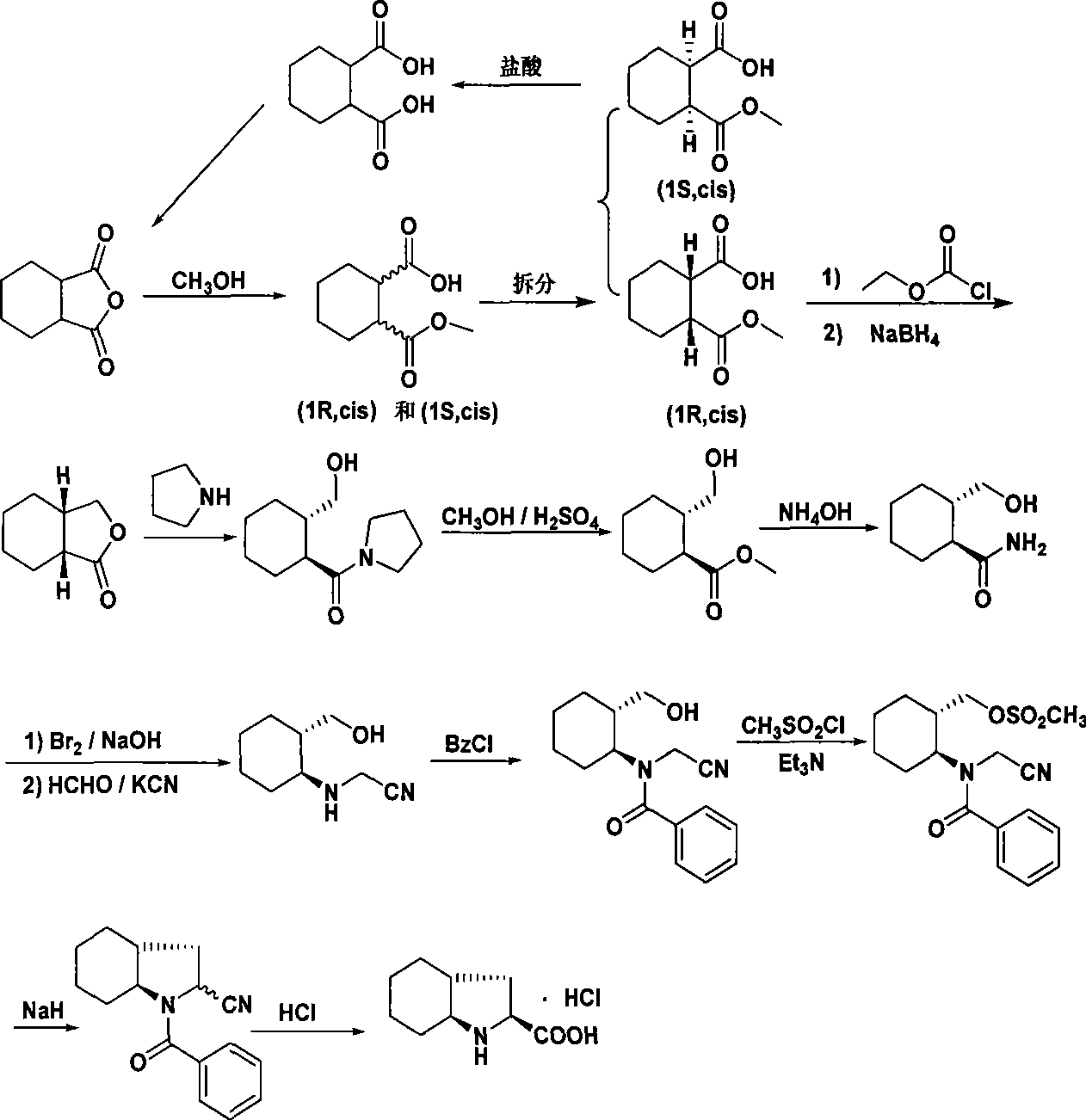
[0027] The synthetic process of Trandolapril key intermediate (2s, 3aR, 7as)-octahydro-1H-indole-2-carboxylic acid is:
[0028] 1. Preparation of ring-opening (mixed monomethyl ester) (FW 186.21)
[0029]
[0030] Add hexahydrophthalic anhydride (30g, 0.2mol) and methanol (300ml) into a 500ml reaction flask, stir at room temperature for 2.5h, concentrate under reduced pressure, add n-hexane (30ml), stir and crystallize, filter with suction and dry , a white solid (33.3 g, yield 92.0%) was obtained with a melting point of 63-65°C.
[0031] 2. Resolution preparation (FW 186.21)
[0032]
[0033](dl) Monomethyl 1,2-cyclohexanedicarboxylate (279.3g, 1.5mol) was dissolved in ethyl acetate (4500ml), and (-)N,N-dimethylchlorase amine (252.2g, 1.1mol), stirred evenly, left to stand for crystallization overnight, filtered with suction, collected the filtrate, dissolved the filter cake in ice water (600ml), extracted with ethyl acetate (600ml), collected the separated water laye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com