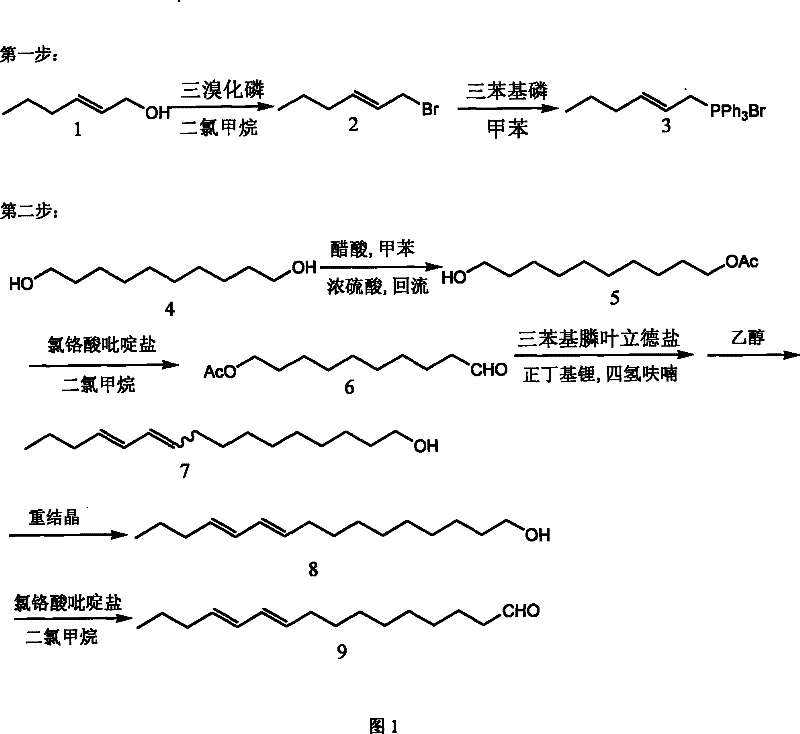
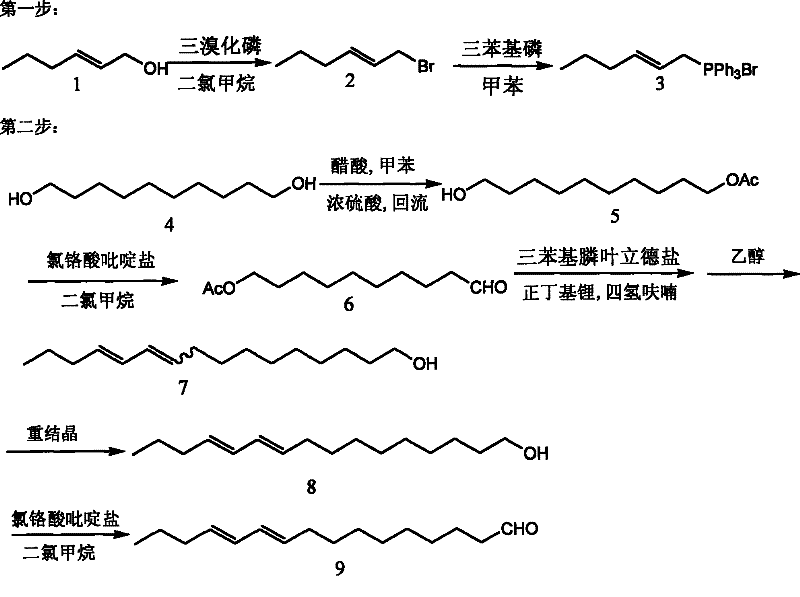
Method for synthesizing compound E10, E12-hexadecadienal in sex pheromone of legume pod borer
A synthesis method and a technology for the properties of P. japonica are applied in the field of synthesis of compounds E10, E12-hexadecadienal in the P. japonicus sex pheromone, and can solve the problem of large residues, excessive pesticide residues, short harvest time, etc. problem, to achieve the effect of simple and safe operation, high utilization rate of raw materials, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] The present invention will be described in further detail below in conjunction with the embodiments.
[0025] 1. Preparation of compound (3) 2-hexenyl triphenylphosphonium bromide: compound (1) trans-2-hexenol was dissolved in dry dichloromethane, cooled to -10°C under nitrogen protection, slowly Add dropwise 0.33% (molar percent) of phosphorus tribromide, continue stirring for 30 minutes after the dropwise addition, quench the reaction with a saturated sodium bicarbonate solution of ice, add a large amount of petroleum ether to dilute, such as 5, or more than 10 times, and then Wash with saturated brine, then collect the organic phase and dry it with anhydrous sodium sulfate for half an hour, remove the sodium sulfate by filtration, and then remove the petroleum ether with a rotary evaporator to obtain compound (2) trans-1-bromo-2-hexyl The crude product of alkene, without further purification, was dissolved in dry toluene, added 1.2% (molar percent) of triphenylphosph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com