Pharmaceutical composition containing lycopene and preparation method thereof
A technology for lycopene and solid pharmaceutical preparations is applied in the field of lycopene-containing pharmaceutical compositions and their preparation, and can solve the problems of low bioavailability, low solubility of lycopene, poor dissolution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
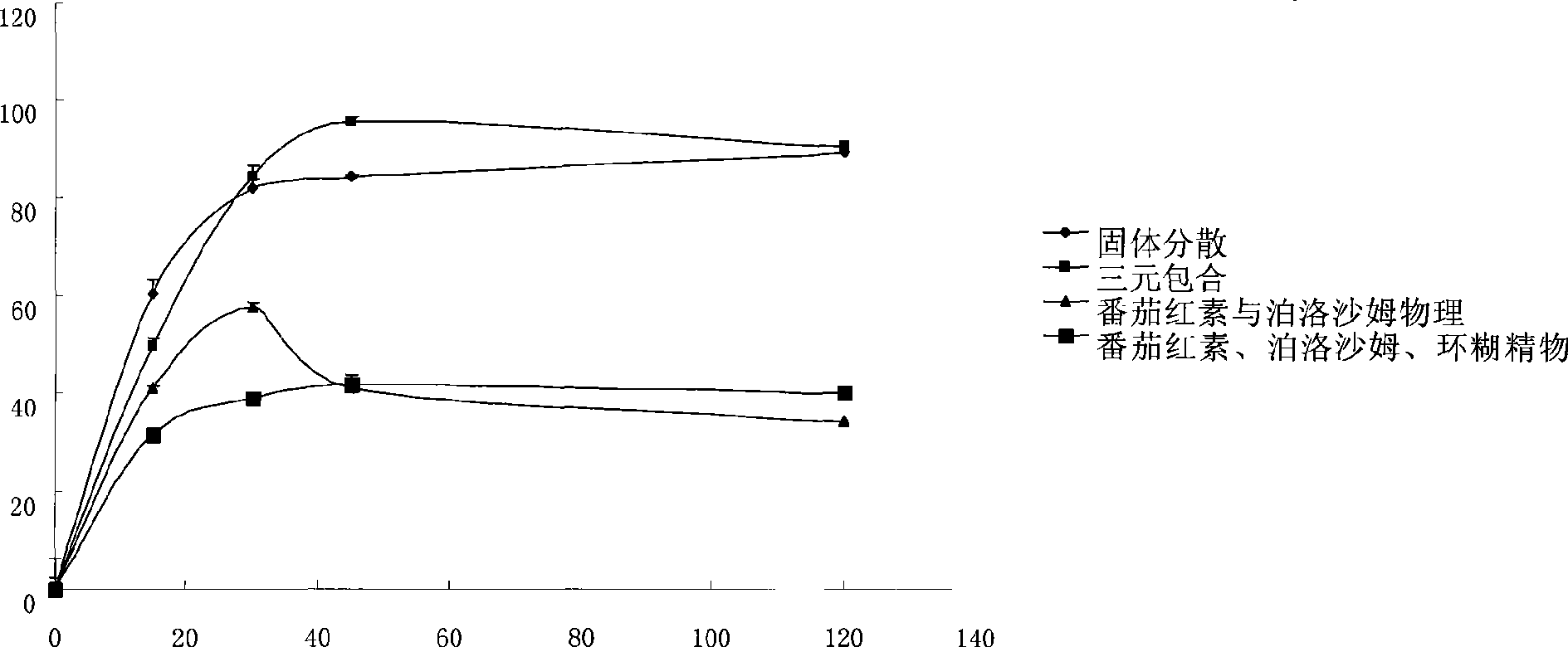
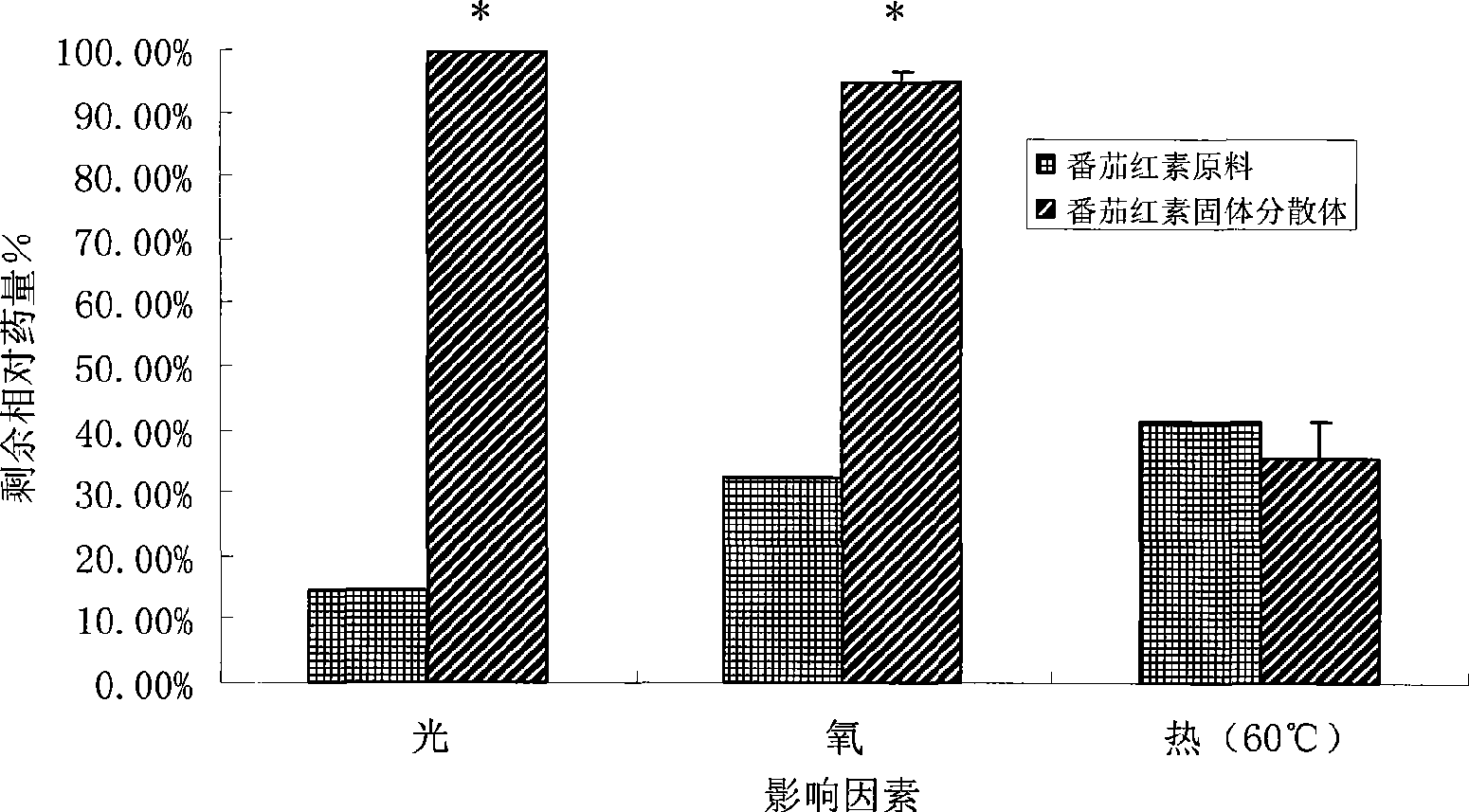
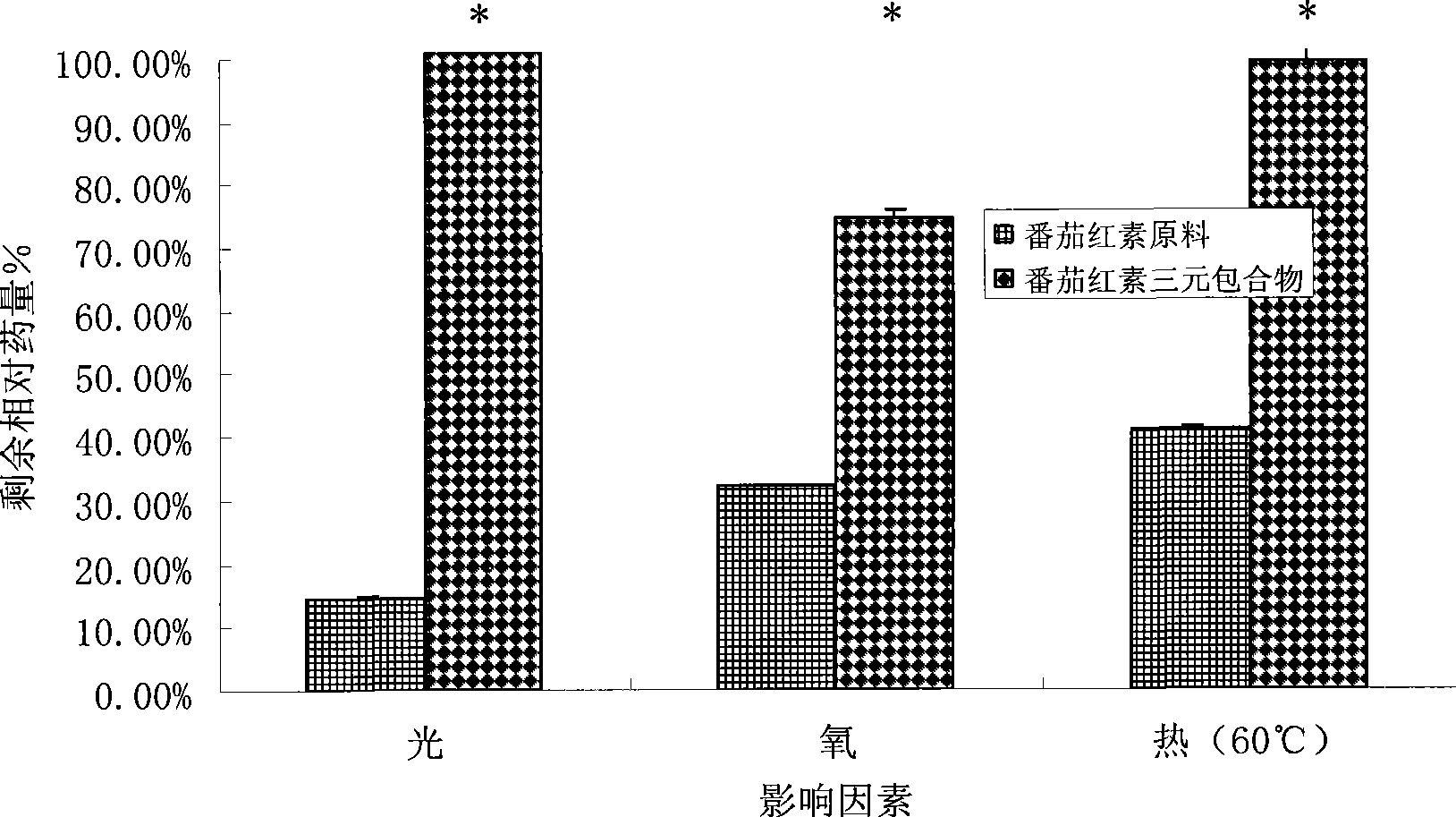
Image
Examples
Embodiment 1
[0045] Preparation of Lycopene Solid Dispersion and Its Tablets
[0046] Take 5g of lycopene and 1885g of poloxamer, add 600ml of tetrahydrofuran to ultrasound to dissolve, evaporate most of the solvent on a rotary evaporator, water bath temperature of 40℃, put it in vacuum for 24h, grind the dried product to obtain a solid dispersion .
[0047] Mix 256 g of lactose with the solid dispersion first, then add 5.6 g of differential silica gel, 2.8 g of cross-linked polyvinylpyrrolidone, and 5.6 g of magnesium stearate, mix well, and press 1000 tablets.
[0048] The tablets dissolve 75% in water in 45 minutes (n=6).
Embodiment 2
[0050] Preparation of Lycopene Solid Dispersion and Its Tablets
[0051] Take 5g of lycopene and 18815g of poloxamer, add 600ml of tetrahydrofuran to ultrasonically dissolve, and evaporate most of the solvent on a rotary evaporator. The temperature of the water bath is 40℃, and it is dried in vacuum for 24h. The dried product is ground to obtain a solid dispersion. .
[0052] Mix 251.6g of lactose with the solid dispersion first, 2.8g of cross-linked polyvinylpyrrolidone and 5.6g of magnesium stearate, mix evenly, and press 1000 tablets.
[0053] The tablets dissolve 76% in water in 45 minutes (n=6).
Embodiment 3
[0055] Preparation of Lycopene Solid Dispersion and Its Tablets
[0056] Take 5g of lycopene and 18825g of poloxamer, add 600ml of tetrahydrofuran to ultrasonically dissolve, evaporate most of the solvent on a rotary evaporator, water bath temperature of 40℃, put it in vacuum for 24h, grind the dried product to obtain a solid dispersion .
[0057] Mix 241.6g of lactose with the solid dispersion, dry granulation and granulate, add 2.8g of cross-linked polyvinylpyrrolidone and 5.6g of magnesium stearate, mix well, and press 1000 tablets.
[0058] The tablet can dissolve 83% in water in 45 minutes (n=6).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com