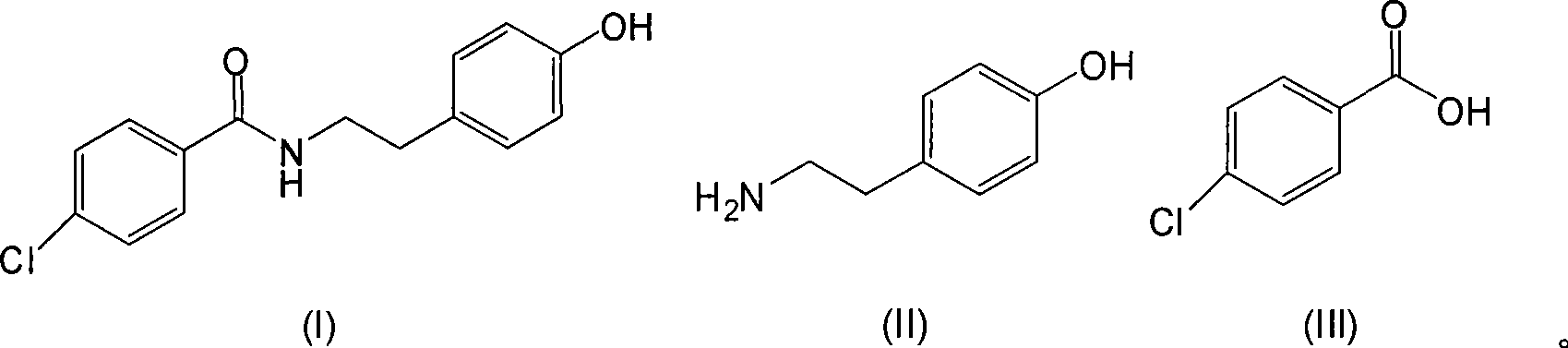
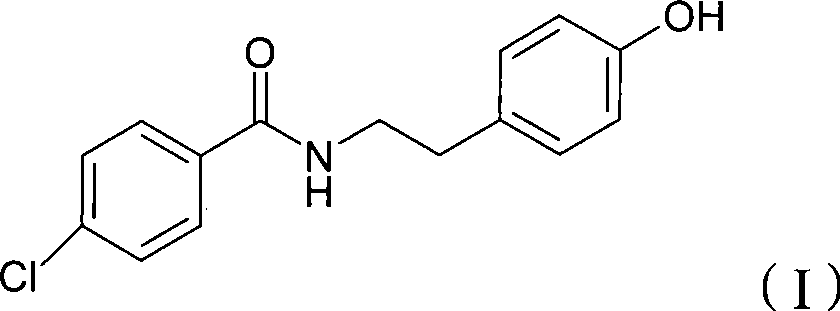
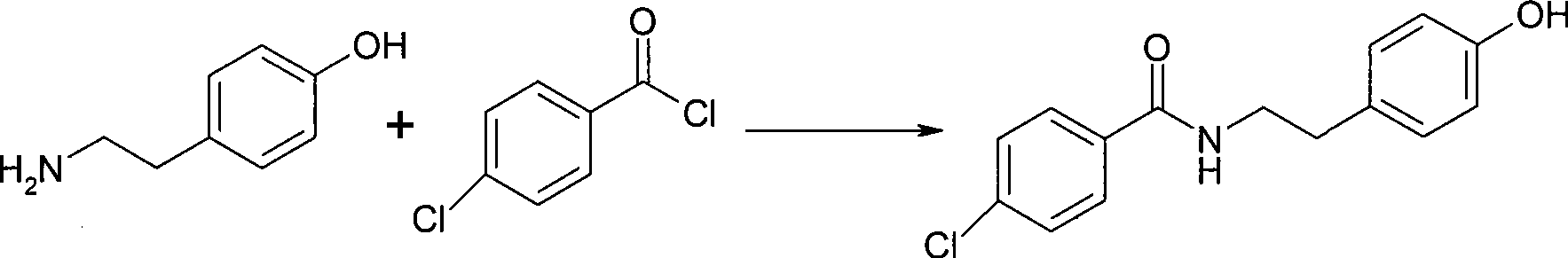
Chemical synthesis method of N-(4-chlorobenzoyl)-tyramine
A chlorobenzoyl, chemical synthesis technology, applied in the field of chemical synthesis of N--tyramine, can solve the problems of high sealing requirements, high cost, corrosion and other problems of reaction equipment, achieve great implementation value and social and economic benefits, eliminate Large hidden dangers in process safety, simple and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 50g of tyramine, 5g of boric acid, 58g of p-chlorobenzoic acid, 460ml of xylene into a clean 1000ml flask equipped with a water separator, stir and heat to reflux with water for 9.5 hours; distill xylene under reduced pressure; add 75ml of ethanol , heating to reflux for beating for 1 hour; cooling and suction filtration; drying to obtain 95.3 g of the target product with a yield of 95.1%.
Embodiment 2
[0035] Add 40g of tyramine, 4g of metaboric acid, 50g of p-chlorobenzoic acid, and 460ml of xylene into a clean 1000ml flask equipped with a water separator, stir and heat under reflux with water for 8 hours; distill xylene under reduced pressure; add ethanol 75ml, heated to reflux for beating for 1 hour; cooled and suction filtered; dried to obtain 74.9g of the target product with a yield of 93.4%.
Embodiment 3
[0037] Add 30g of tyramine, 3g of boric acid, 41g of p-chlorobenzoic acid, 420ml of xylene into a clean 1000ml flask equipped with a water separator, stir and heat under reflux with water for 12 hours; distill xylene under reduced pressure; add 75ml of ethanol , heated to reflux for beating for 1 hour; cooled and suction filtered; dried to obtain 56.9 g of the target product with a yield of 94.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com