Polymer-bound tripe amino-acid schiff base metal copper complexes catalyst and synthesis method thereof
A technology of amino acids and Schiff bases, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
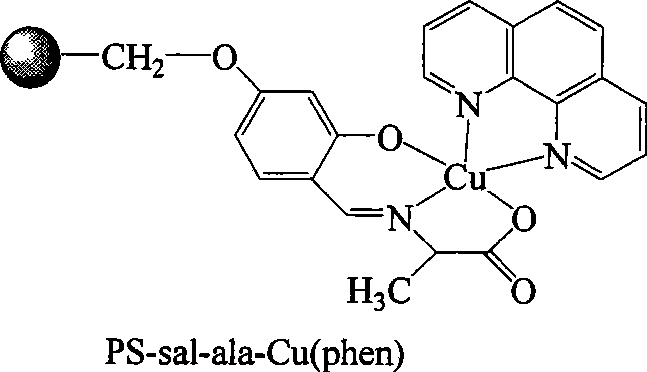
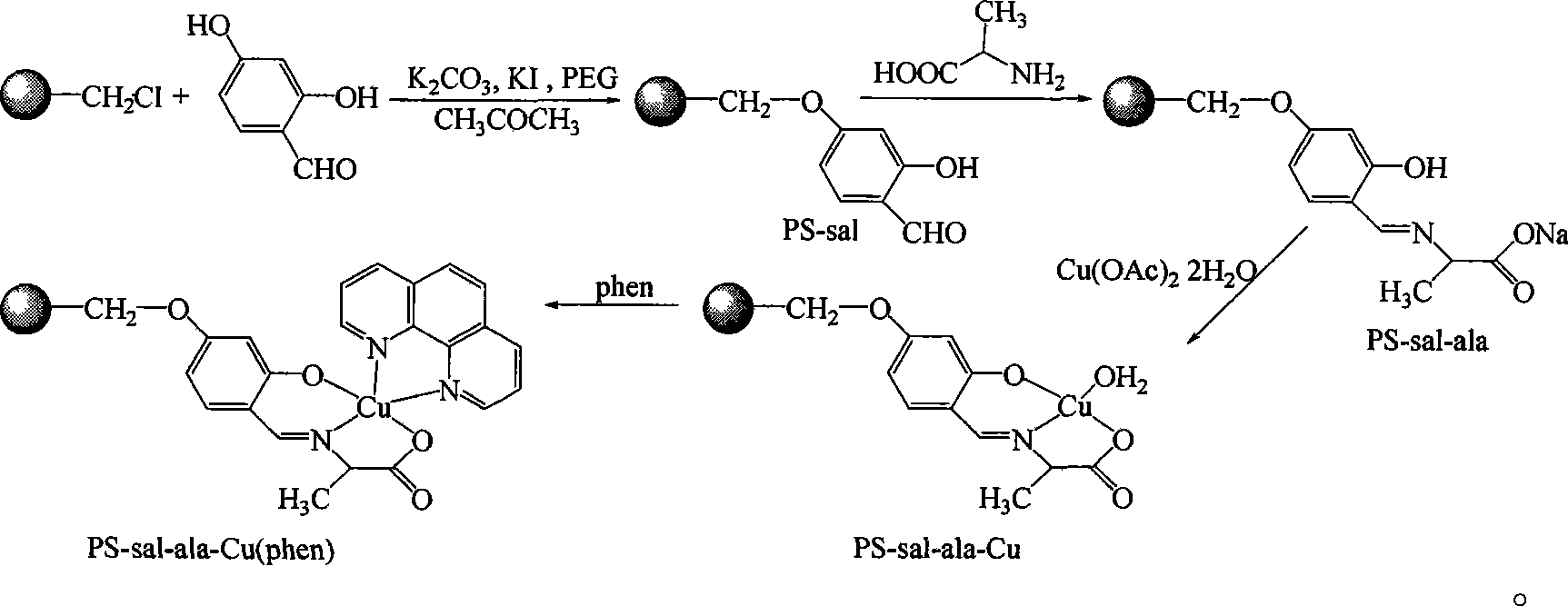
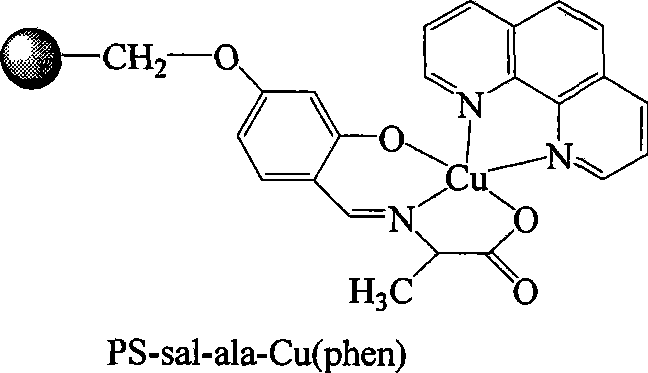
[0019] ①Add 3g of chloromethyl polystyrene to 100ml of acetone, heat and swell at 60°C for 12 hours, then add 1.4g of 2,4-dihydroxybenzaldehyde, 1.38g of anhydrous potassium carbonate, 0.16g of potassium iodide, and 0.2ml of PEG-400 and 50ml of acetone solvent, and the above reactants were heated to 60 ° C, under the protection of nitrogen, stirred and reacted for 12 hours, filtered the solid product, and washed 5 times with 20ml of acetone respectively to obtain PS-sal, which was set aside;
[0020] ② Dissolve 0.89g of alanine in a mixed solution of methanol and water, the volume ratio of methanol to water is 1:1, add 0.4g of sodium hydroxide and stir to dissolve the sodium hydroxide, then add the 4.4 gPS-sal, and heated the reactant to 55°C-60°C, reacted for 12 hours under stirring to obtain a light yellow solid suspension, filtered the solid product, and washed several times with methanol and water respectively to obtain PS-sal-ala, ;
[0021] ③Dissolve 1.99g of copper ace...
example 1
[0025] Under normal pressure, when the temperature is 105°C-115°C, the polymer-supported ternary amino acid Schiff base metal copper complex catalyst and ethylbenzene (2mg: 2ml) are added to the reactor, and no additives are added. Under the conditions, molecular oxygen oxidizes ethylbenzene, the conversion rate of ethylbenzene is 20%, the selectivity of product acetophenone is 100%, and no by-product is formed.
example 2
[0027] Under normal pressure, when the temperature is 95°C-105°C, the polymer-supported ternary amino acid Schiff base metal copper complex catalyst and ethylbenzene (2mg: 2ml) are added to the reactor, and no additives are added. Under the conditions, molecular oxygen oxidizes ethylbenzene, the conversion rate of ethylbenzene is 12%, and the selectivity of product acetophenone is 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com