Metal nanoparticles stabilized with a carboxylic acid-organoamine complex
A technology of metal nanoparticles and amine complexes, which is applied in nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, and can solve problems such as dimensional stability and plastic substrates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Sodium hydroxide solution (50 mL) was added to a butyric acid solution (50 mL) in methanol. After stirring the mixture for 10 minutes, silver nitrate (9.86 g, 0.058 mol) in distilled water (50 mL) was added, forming a white precipitate of silver propionate. After the precipitate was filtered, washed with distilled water and methanol, and dried in vacuo, white silver butyrate (10 g) was obtained, wherein the percent yield of the previous reaction was 90.7%.
[0093] Silver butyrate (1.95 g, 10 mmol) and 1-hexadecylamine (6.04 g, 25 mmol) were dissolved in 20 mL of toluene by heating the mixture to 50°C until the silver butyrate was completely dissolved. This dissolution occurs within approximately 5 minutes. To this solution was added a solution of phenylhydrazine (0.595 g, 5.5 mmol) in toluene (10 mL) and stirred for 5 minutes. The resulting reaction mixture was stirred at 50°C for an additional 30 minutes before cooling to room temperature. Next, the mixture was add...
Embodiment 2-5
[0097] In Examples 2-5, the procedure used in Example 1 was followed except that in Example 2 (valeric acid), Example 3 (hexanoic acid), Example 4 (octanoic acid) and Example 5 (undecene acid) using different carboxylic acids. In addition, Examples 4 and 5 used a different organic amine (dodecylamine).
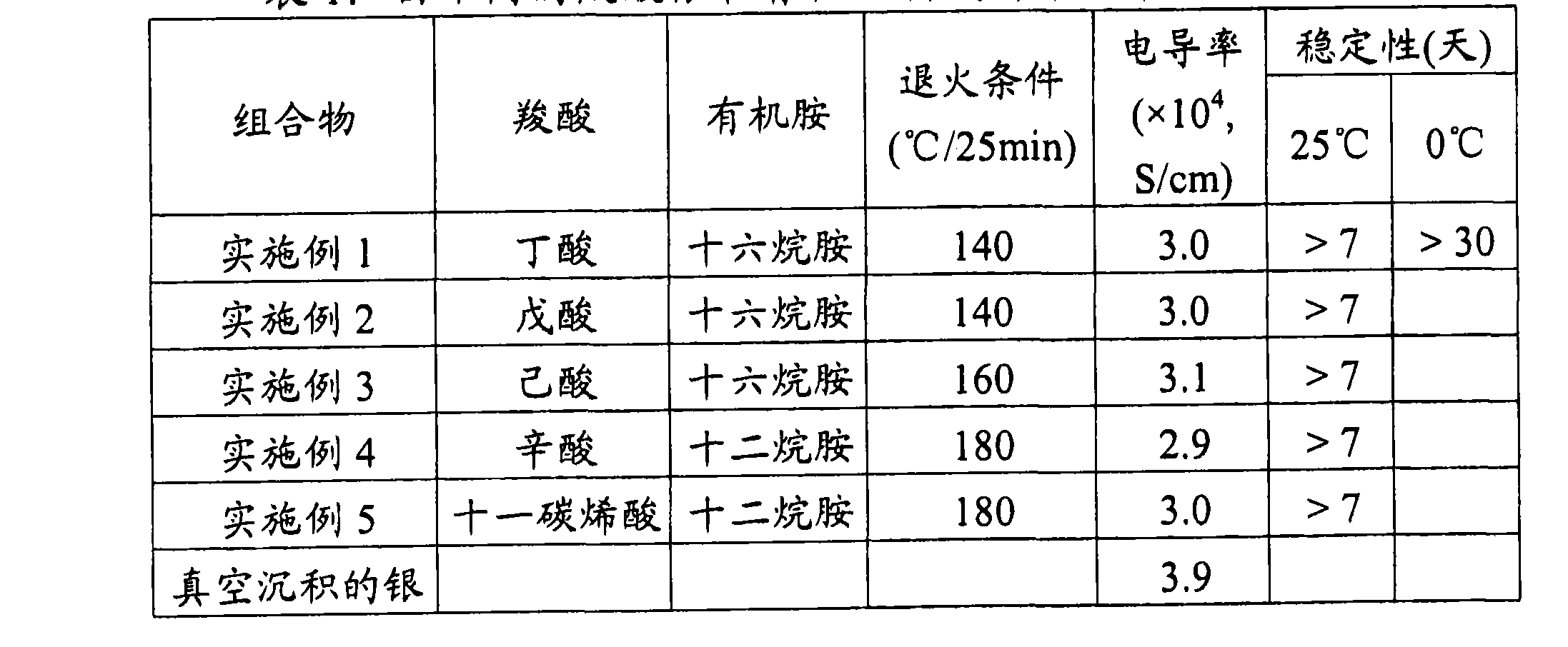
[0098] Table 1: Properties of silver nanoparticles made from different silver carboxylates and organic amines
[0099]
[0100] Table 1 shows that, depending on temperature, silver nanoparticles with different carboxylic acid-organic amine stabilizers are extremely stable over a period of 7 to 13 days and can be converted to high Conductive film, the conductivity is 2.9×10 4 to 3.9×10 4 S / cm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com