Method for preparing unsymmetrical biphase composite oxygen permeable membrane
An oxygen-permeable membrane and asymmetric technology, which is applied in the field of preparation of asymmetric dual-phase composite oxygen-permeable membranes, can solve the problems of pinholes or cracks, difficulty in densification, and complicated processes, and achieve high structural stability and high oxygen permeability. , the effect of high chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
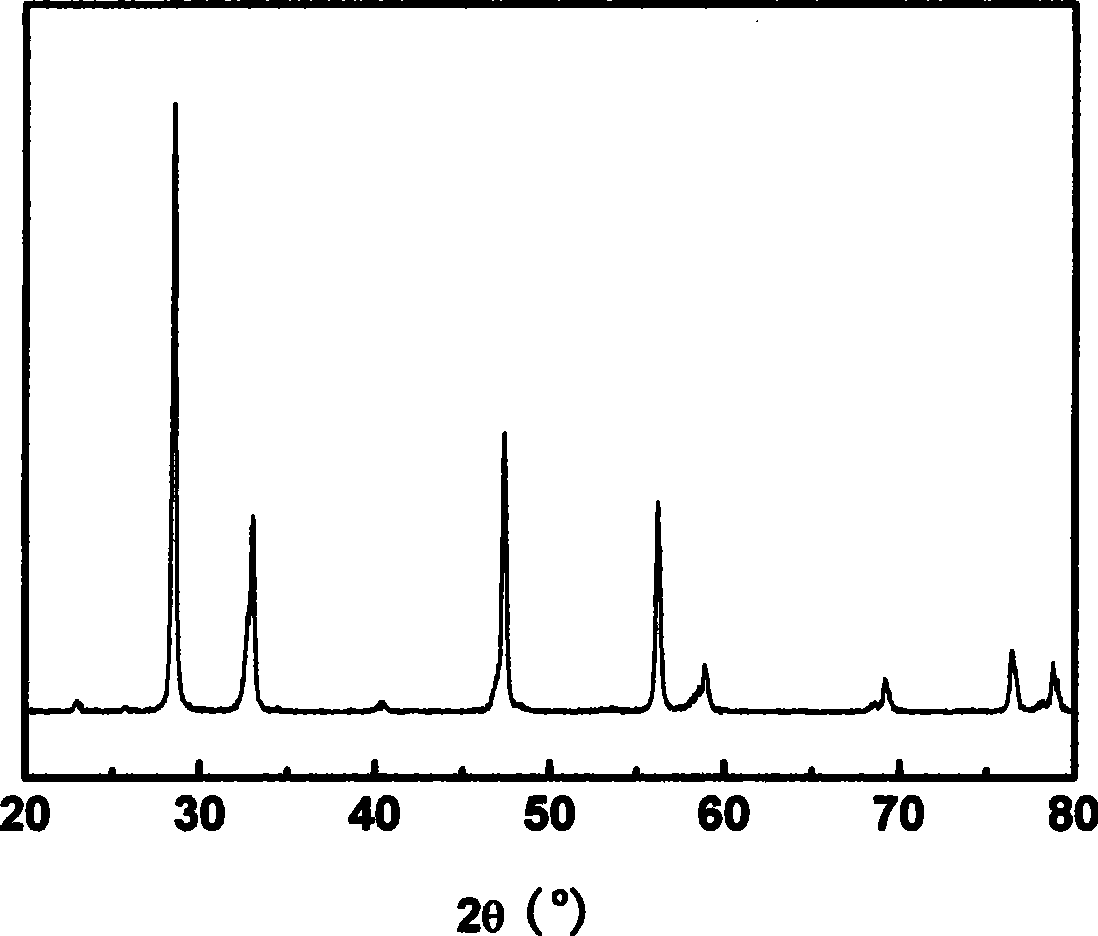
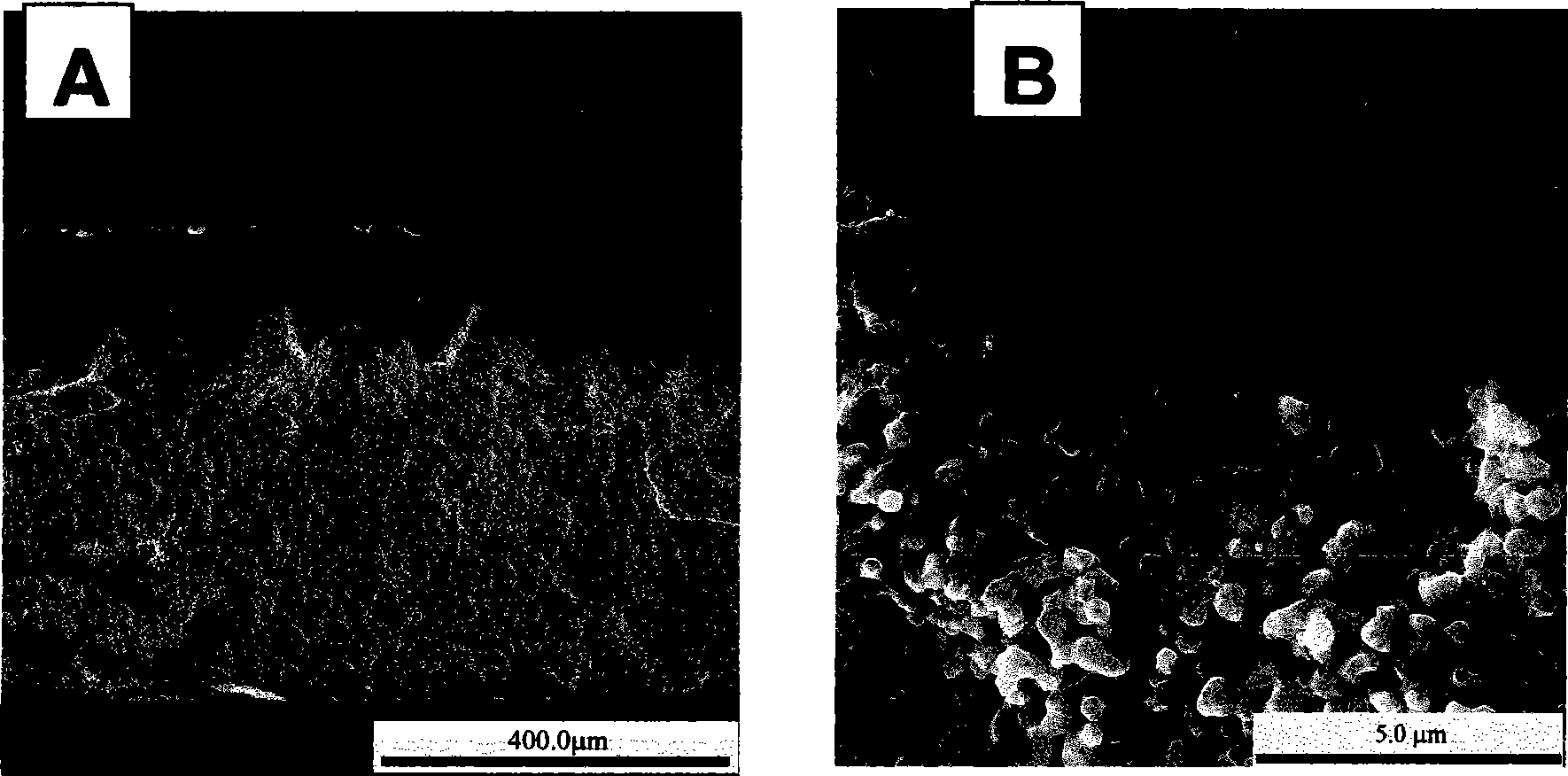
[0026] First mix all metal ion nitrates to synthesize 65wt% Ce 0.85 SM 0.15 o 1.925 —35wt%Sm 0.6 Sr 0.4 FeO 3 Two-phase composite membrane, and then prepare an asymmetric two-phase composite oxygen-permeable membrane by dissolving in hydrochloric acid. The specific steps are as follows: according to the relative mass ratio of the desired two kinds of oxides, all metal ions (Ce 3+ 、Sm 3+ 、Sr 2+ , Fe 3+ ) Nitrate is poured into a beaker, and an appropriate amount of EDTA and citric acid are added thereto, that is, the ratio of the amount of EDTA and citric acid to the total metal ion is 1:1 and 1:1.5, respectively. Then use NH 3 ·H 2 O adjust the pH of the solution to 6.0, heat and stir the solution at a constant temperature of 80°C, and finally obtain a colloid with the continuous evaporation of water, pretreat the colloid at 400°C for preliminary decomposition, and calcinate the primary powder at 950°C for 5 hours That is, the composite oxide powder obtained by the ...
Embodiment 2
[0028] First mix all metal ion nitrates to synthesize 65wt% Ce 0.80 SM 0.20 o 1.9 —35wt%Sm 0.5 Sr 0.5 FeO 3 Two-phase composite membrane, and then prepare an asymmetric two-phase composite oxygen-permeable membrane by controlling the dissolution time of hydrochloric acid. The specific method is as follows: according to the relative mass ratio of the desired two kinds of oxides, all metal ions (Ce 3+ 、Sm 3+ 、Sr 2+ , Fe 3+ ) Nitrate is poured into a beaker, and an appropriate amount of EDTA and citric acid are added thereto, that is, the ratio of the amount of EDTA and citric acid to the total metal ion is 1:1 and 1:1.5, respectively. Then use NH 3 ·H 2 O adjust the pH of the solution to 6.0, heat and stir the solution at a constant temperature of 80°C, and finally obtain a colloid with the continuous evaporation of water, pretreat the colloid at 400°C for preliminary decomposition, and calcinate the primary powder at 950°C for 5 hours That is, the composite oxide pow...
Embodiment 3
[0030] First mix all metal ion nitrates to synthesize 75wt% Ce 0.80 SM 0.2 0O 1.9 —25wt%Sm 0.5 Sr 0.5 FeO 3-δ Two-phase composite membrane, and then prepare an asymmetric two-phase composite oxygen-permeable membrane by controlling the concentration of hydrochloric acid. The specific method is as follows: according to the relative mass ratio of the desired two oxides, all metal ions (Ce 3+ 、Sm 3+ 、Sr 2+ , Fe 3+ ) Nitrate is poured into a beaker, and an appropriate amount of EDTA and citric acid are added thereto, that is, the ratio of the amount of EDTA and citric acid to the total metal ion is 1:1 and 1:1.5, respectively. Then use NH 3 ·H 2 O adjust the pH of the solution to 6.0, heat and stir the solution at a constant temperature of 80°C, and finally obtain a colloid with the continuous evaporation of water, pretreat the colloid at 400°C for preliminary decomposition, and calcinate the primary powder at 950°C for 5 hours That is, the composite oxide powder obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com