Chemical semi-synthetic process of 10-hydroxycamptothecin
A technology for hydroxycamptothecin and camptothecin, which is applied in the field of chemical semi-synthesis of 10-hydroxycamptothecin, can solve the problems of long time consumption, many final steps, large amount of extraction solvent, etc., and achieves production cost saving, The effect of reducing the risk factor and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of palladium carbon
[0027] Wash 100 g of 4-8 mesh activated carbon with 1 mol / L nitric acid for 5 hours, wash with water until neutral, and dry at 100° C. for 10 hours to obtain a carrier of activated carbon for future use. Take 3.2 g of palladium acetate solution containing 16% palladium, add deionized water to 40 mL, add 10% aqueous sodium hydroxide solution to adjust the pH of the palladium acetate solution to 5-7, and after the palladium acetate solution is stable for 1 hour, the above-mentioned treated Activated carbon was impregnated with palladium acetate solution for 3h to obtain the catalyst precursor. The catalyst precursor was aged for 24 hours, treated with high-purity hydrogen for 5 hours, filtered under the protection of hydrogen, and washed with water until neutral. Catalyst at 180°C and vacuum degree 1.013×10 -3 It was dried under Pa for 72h, then dropped to room temperature under nitrogen protection and released from th...
Embodiment 2
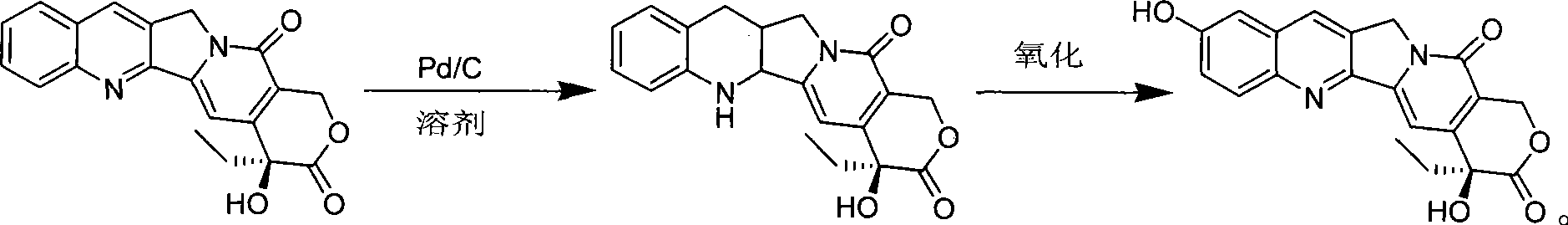
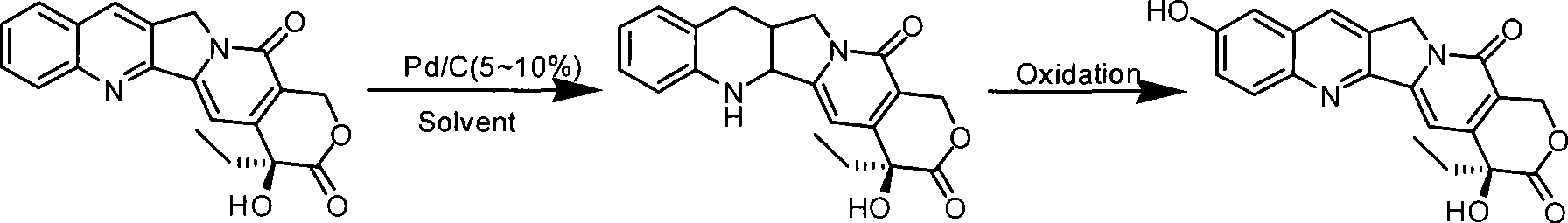
[0029] Add 200 g of camptothecin and 120 g of 5% Pd / C into a 5 L three-necked round bottom flask, and add 3 L of 1,4-dioxane. Under normal pressure and stirring, pass through hydrogen gas and heat to 80°C, and react for 24 hours. After the reaction is finished, the reaction solution is cooled, and the reacted catalyst is removed by filtration, and the reaction solution is directly used for the next oxidation reaction.
[0030] Transfer the above 1,4-dioxane reaction solution (about 3.1L) to a 20L reaction flask, add an equal amount of water to dilute the reaction solution, and stir vigorously at room temperature (about 40°C), and pour into the reaction solution within 2h 320g IBX (2.0eq) was added in 5 batches, and the addition was completed within 2h. Then 158 g (1.0 eq) of IBX were added in three batches one hour apart. After the oxidant was added, the product was filtered after stirring for 12 hours, rinsed with methanol, and dried to obtain about 119 g of the product (wh...
Embodiment 3
[0032] Add 200 g of camptothecin and 100 g of 10% Pd / C into a 5 L three-necked round bottom flask, and add 3 L of tetrahydrofuran. Under normal pressure and stirring, pass through hydrogen and heat to 60°C, and react for 24 hours. After the reaction is finished, the reaction solution is cooled, and the reacted catalyst is removed by filtration, and the reaction solution is directly used for the next oxidation reaction.
[0033] Transfer the above tetrahydrofuran reaction solution (about 3.1L) to a 20L reaction flask, add an equal amount of water to dilute the reaction solution, and stir vigorously at room temperature (about 20°C), add 320g IBX in 5 batches to the reaction solution within 2h (2.0eq), 2h added. 176 g (1.1 eq) of IBX were then added in three batches one hour apart. After the oxidant was added, the product was obtained by filtration after stirring for 12 hours, rinsed with methanol, and dried to obtain about 72 g of the product (wherein the HCPT content of the p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com