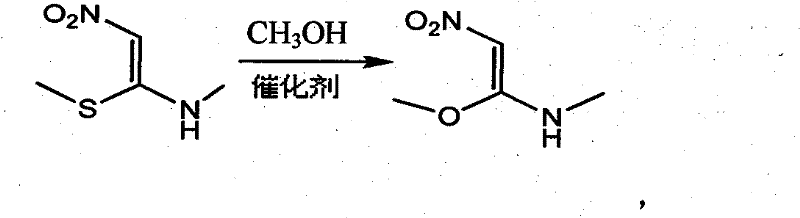
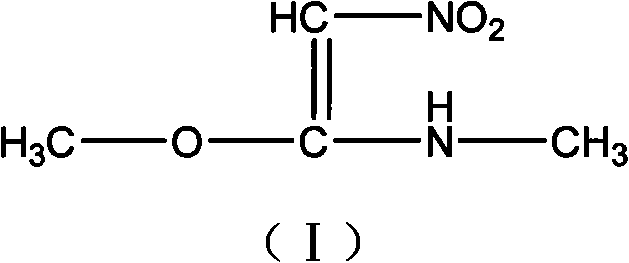
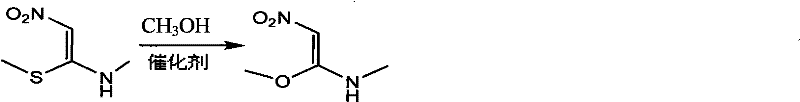Process for synthesizing 1- methylamino-1-methoxy-2-nitroethylene
A technology of nitroethylene and a synthesis method, applied in the field of compounds, can solve the problems of complicated operation, long reaction time, expensive trimethyloxonium tetrafluoroborate, etc., and achieves improved operation safety, reduced labor intensity, and shortened production. effect of cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 500ml of anhydrous methanol to a 1000ml three-necked round-bottomed flask with a magnetic stirrer, reflux condenser and thermometer, add 2.3g (0.1mol) of sodium metal under stirring, and then add 149g (1mol) after the sodium metal is dissolved. 1-Methylamino-1-methylthio-2-nitroethylene, stir and heat up to 60°C, react for 2-5 hours, evaporate methanol to dryness under reduced pressure to obtain a yellow solid, directly add 1500ml of absolute ethanol and heat up to reflux for 1h, Cool to below room temperature, filter, and wash the filter cake once with absolute ethanol to obtain 80.5 g of 1-methylamino-1-methoxy-2-nitroethylene as a light yellow solid, with a yield of 61% and a purity of 98.5% .
Embodiment 2
[0023] Add 500ml of anhydrous methanol to a 1000ml three-necked round-bottom flask with a magnetic stirrer, reflux condenser and thermometer, add 4g of metal potassium under stirring, and then add 149g of 1-methylamino-1-methylsulfide after the metal sodium is dissolved Base-2-nitroethylene, stir and heat up to 60°C, react for 2-5h, evaporate methanol to dryness under reduced pressure to obtain a yellow solid, directly add 1500ml of absolute ethanol, heat up to reflux for 1h, cool to below room temperature, filter, filter cake Wash once with absolute ethanol to obtain 1-methylamino-1-methoxy-2-nitroethylene as a light yellow solid.
Embodiment 3
[0025] Add 4500ml of anhydrous methanol to a 5000ml four-necked round bottom flask with a magnetic stirrer, reflux condenser and thermometer, add 56.7g (0.15mol) of sodium methoxide under stirring, and then add 1043g (7mol) after the sodium methoxide is dissolved. 1-Methylamino-1-methylthio-2-nitroethylene, stir and heat up to reflux, react for 2-5 hours, evaporate methanol to dryness under reduced pressure to obtain a yellow solid, directly add 1500ml of absolute ethanol, heat up to reflux for 1h, cool After cooling down to room temperature, filter and wash the filter cake once with absolute ethanol to obtain 600.5 g of 1-methylamino-1-methoxy-2-nitroethylene as a light yellow solid with a yield of 65%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com