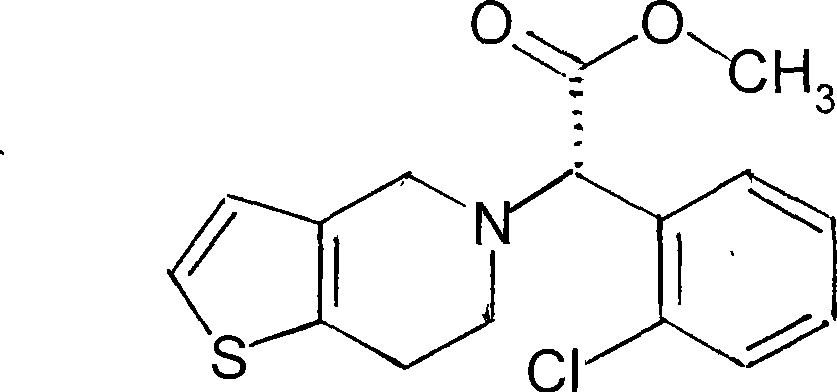
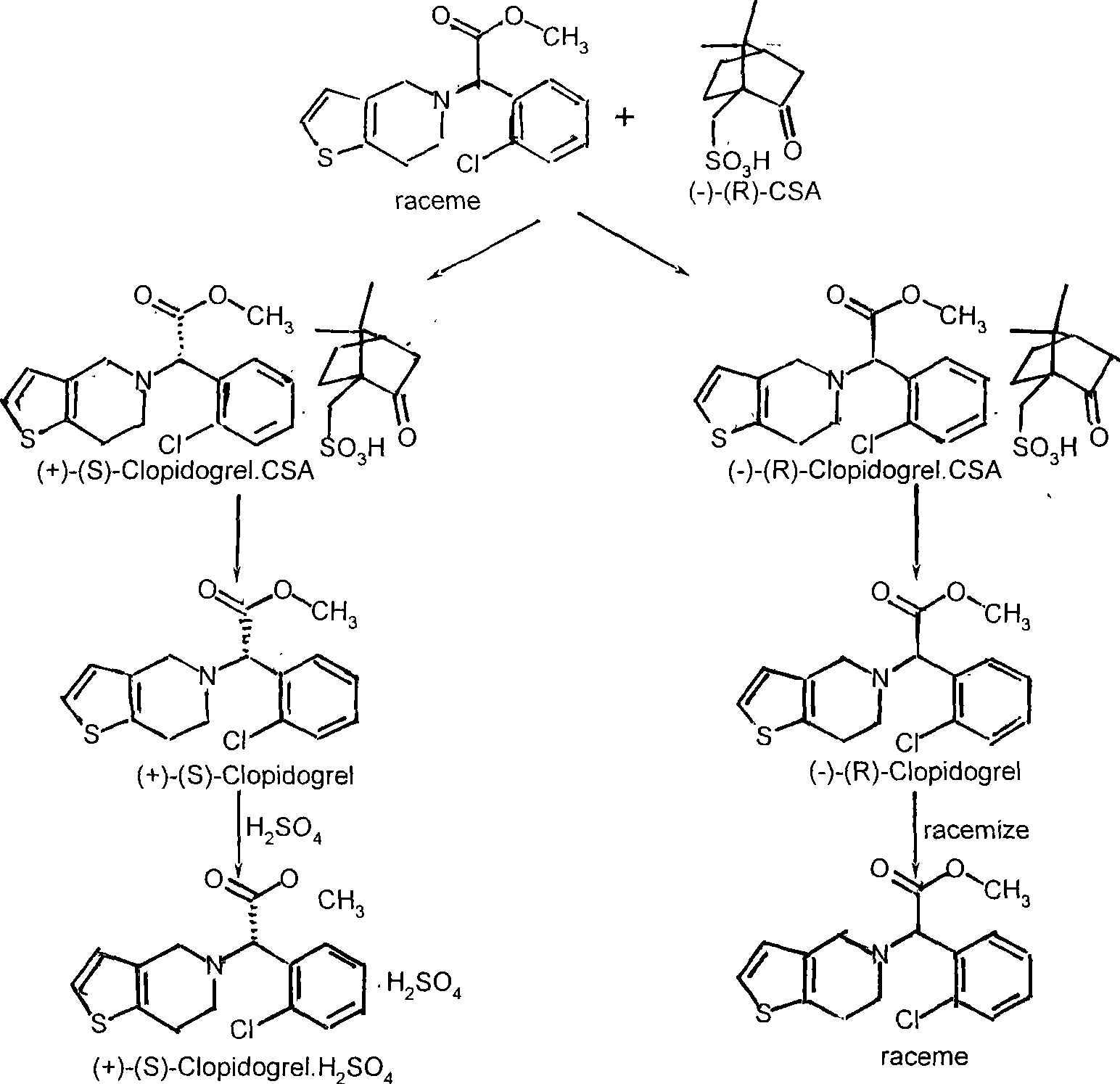
Production method of (-)-(R)- clopidogrel (-)-(R)-camphorsulfonate racemisation
A technology of camphorsulfonate and production method, which is applied in the field of drug synthesis, can solve the problems of unfavorable industrial production, complex reaction, high cost, etc., and achieve the effects of stable quality, simple preparation method and improved yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0034]Add 32.1g (0.1mol) of racemic clopidogrel free base, 320ml of acetone (10 times) into the reaction flask, stir until completely dissolved, add 18.6g (0.08mol) (-)-(R)-camphorsulfonic acid ( 0.8 times), react at 30°C for 1 hour, crystallize at 0°C for 12 hours, heat up to 50°C, hold for 2 hours, crystallize at 0°C for 2 hours, filter, rinse with acetone to obtain (+)-(S)-chlorine Pidogrel (-)-(R)-camphorsulfonate 22.0 g, HPLC 99%, melting point 161-163°C, [α]=+24.7°, yield 39.67%.
Embodiment 3
[0036] Preparation of (+)-(S)-Clopidogrel Sulfate
[0037] (+)-(S)-clopidogrel (-)-(R)-camphorsulfonate 22.0 g (0.040 mol) in Reference Example 2, 176 ml of ethyl acetate and 176 ml of water were added to the reaction flask, saturated with NaHCO 3 The aqueous solution was adjusted to neutral pH, the ethyl acetate layer was separated, the aqueous layer was extracted with 50 ml of ethyl acetate, the ethyl acetate layers were combined, washed with 50 ml of saturated aqueous NaCl solution, and anhydrous MgSO 4 Dry, concentrate to dryness under reduced pressure to obtain 12.5 g of (+)-(S)-clopidogrel free base oil, add 62.5 ml of isopropanol (5 times), and dropwise add 2.6 ml of concentrated sulfuric acid (1.3 times) at 10°C , crystallized at 0°C for 8 hours, filtered and rinsed with acetone to obtain (+)-(S)-clopidogrel sulfate 16.4g, HPLC 99.3%, melting point 183-184°C, [α]=55.8°, yield 97.55%.
preparation Embodiment 1
[0039] Racemized (-)-(R)-clopidogrel (-)-(R)-camphorsulfonate mother liquor
[0040] About 350 ml of the (-)-(R)-clopidogrel (-)-(R)-camphorsulfonate mother liquor remaining in Reference Example 2 was concentrated to dryness under reduced pressure to obtain 23.3 g of pale yellow oil, and 116.5 ml was added. Methanol (5 times) was stirred until dissolved, 11.7g (weight ratio 1:0.5) sodium methoxide was added, reacted at 30°C for 1 hour, 100ml of ethyl acetate and 100ml of water were added, the organic layer was separated, and the aqueous layer was extracted with 50ml of ethyl acetate , the organic layers were combined, washed with saturated aqueous NaCl, anhydrous MgSO 4 Dry, concentrate to dryness under reduced pressure to obtain 18.4 g of racemic clopidogrel free base oil, add 92 ml of isopropanol (5 times), add 3.9 ml of concentrated sulfuric acid (1.3 times) dropwise at 10 °C, and crystallize 8 at 0 °C hour, filtered, rinsed with acetone to obtain 18.9 g of racemic clopido...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com