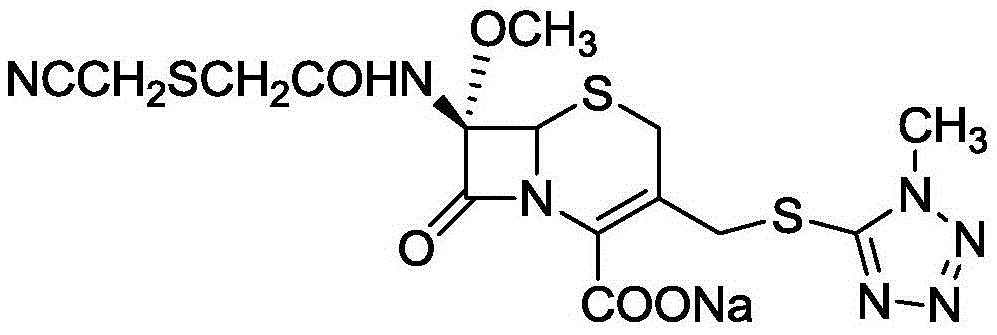
Cefmetazole sodium for reducing anaphylaxis and preparation thereof
A technology for cefmetazole sodium and allergic reactions, applied in medical preparations containing active ingredients, organic active ingredients, organic chemistry, etc., can solve the problems of long production cycle, low product purity, high content of related substances, etc., and improve product quality. High quality, high purity, and good appearance formability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] In a dry and clean three-neck flask, add 50g of 7-MAC, 600ml of chloroform and stir to dissolve, cool to -15°C, add 14g of pyridine, dropwise add 18g of cyanomethylmercaptoacetyl chloride, after the addition is complete, stir for 1 hour, add purified water: Sodium: 40ml of the mixed solution of concentrated hydrochloric acid (9:1:2) was used to terminate the reaction, and the layers were allowed to stand, and the organic phase was taken, dried, and concentrated to obtain 57.5g of cefmetazole diphenylmethyl ester, with a molar yield of 94.7%, HPLC purity 99.35%.
[0040]Cefmetazole diphenylmethyl ester purity test conditions (the same below): use octadecylsilane bonded silica gel as filler; use phosphate buffer (take 2.72g of potassium dihydrogen phosphate, add water to dissolve and dilute to 1000ml)- Acetonitrile (50:50) is the mobile phase; the detection wavelength is 214nm, and the resolution of the Mz-2 peak and the adjacent impurity peak should meet the requirements...
Embodiment 2
[0045] In a dry and clean three-neck flask, add 50g of 7-MAC, add 600ml of chloroform and stir to dissolve, cool to -15°C, add 10g of dimethylaminopyridine, dropwise add 18g of cyanomethylmercaptoacetyl chloride, after the addition is complete, stir for 1 hour, add the purified Water: Sodium chloride: 45ml of mixed solution of concentrated hydrochloric acid (9:1:2) to terminate the reaction, let stand to separate layers, take the organic phase, dry, and concentrate to obtain 58.1g of cefmetazole diphenylmethyl ester, and the molar yield is 95.7 %, HPLC purity 99.3%.
[0046] Take 800ml of dichloromethane and cool down to 5-10°C, add 45g of aluminum trichloride under stirring, add 115g of anisole dropwise, control the temperature at 15-20°C, and cool down to -20~-25°C after adding; keep stirring and add cephalosporin 50g of methazole diphenylmethyl ester, react for 0.5 hours, add 50ml of a mixture of acetone: water: concentrated hydrochloric acid (weight ratio 8:8.2:1) cooled t...
Embodiment 3
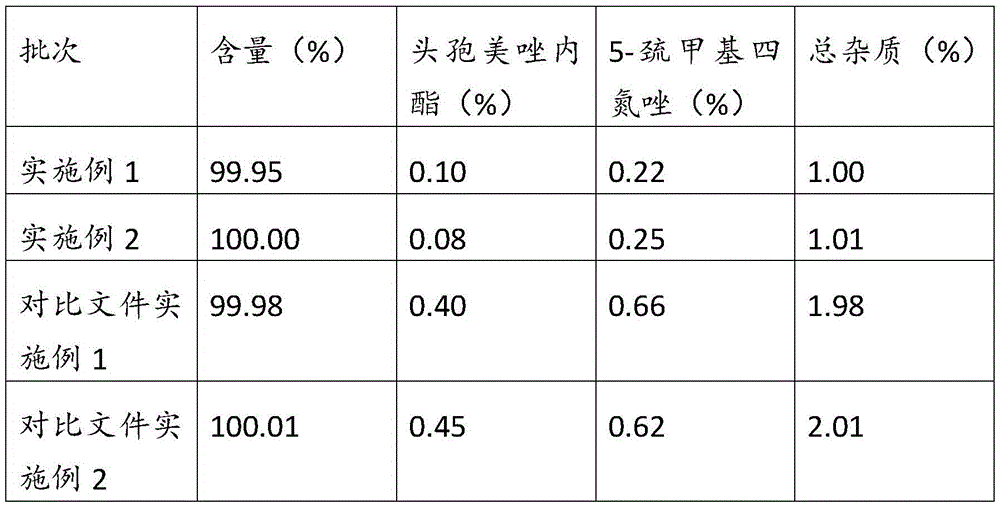
[0048] Embodiment 3, comparative study of product content and key impurities
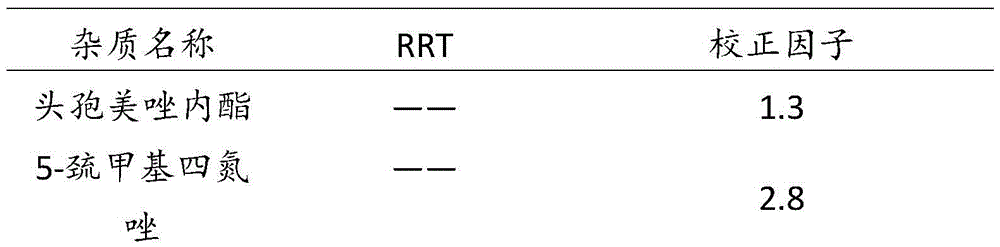
[0049] Get respectively embodiment 1 of the present invention, embodiment 2 and Chinese invention patent CN104557978A embodiment 1 and 2 gained products, refer to the pharmacopoeia standard to measure the key impurity cefmetazolactone in the pharmacopoeia standard of the sample, 5-mercaptomethyl four Nitrozol was tested and the results are shown in the table below:
[0050]
[0051] The results show that, compared with the reference document, in the cefmetazole sodium product obtained in the present invention, the content of key impurities is significantly lower, and the total impurities are far lower than the standard.
[0052] Attachment: Measuring method: Take 20 μl of the control solution and inject it into the liquid chromatograph, adjust the detection sensitivity so that the peak height of the main component chromatographic peak is about 20% of the full scale, and accurately measure the bla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com