Improved preparation method of telavancin or salt thereof
A technology of acid addition salts and compounds, applied in the field of drug synthesis, can solve problems such as short retention time and difficult removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
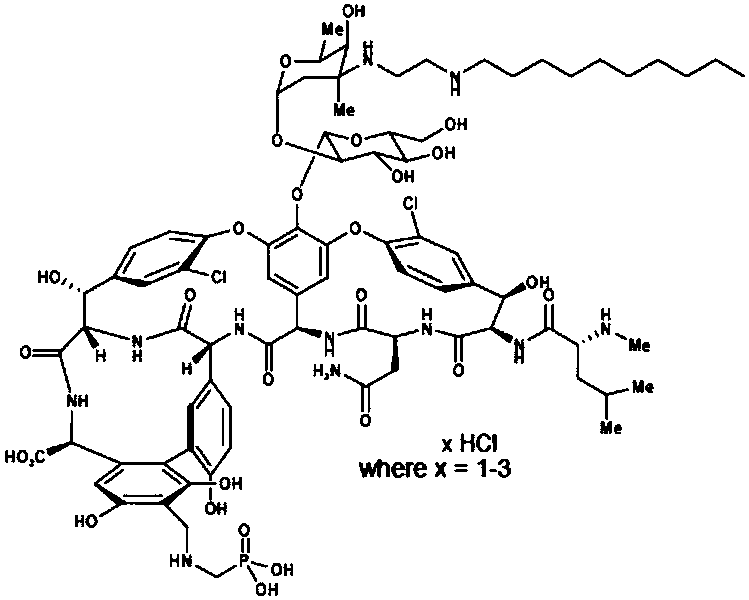
[0049] The preparation of embodiment 1 compound I
[0050]
[0051] Add 40L of N,N-dimethylformamide and 4kg of vancomycin hydrochloride into a 100L reaction tank, stir for 10 minutes, control the temperature of the feed liquid at 0-10°C, and add 1.6L of N,N-diisopropyl Ethylamine, after dropping, stirred for 10 minutes, controlled the temperature of the feed liquid at 0-10°C, and slowly added 1.6 kg of decyl (2-oxoethyl) carbamic acid 9H-fluorene-9-methyl ester (compound of formula II) After the addition is complete, the temperature of the feed liquid is controlled at 5±5° C. and the reaction is stirred for 24 hours. Samples are tracked and detected by HPLC until the end of the reaction.
[0052] Add 16L of anhydrous methanol, control the temperature at 0-10°C, slowly add 1.1L of trifluoroacetic acid dropwise to the feed liquid, after dropping, keep the feed liquid temperature at 5±5°C and stir for 1 hour, add 0.4kg of borane-tert For the butylamine complex, keep the temp...
Embodiment 2
[0054] The preparation of embodiment 2 compound III
[0055]
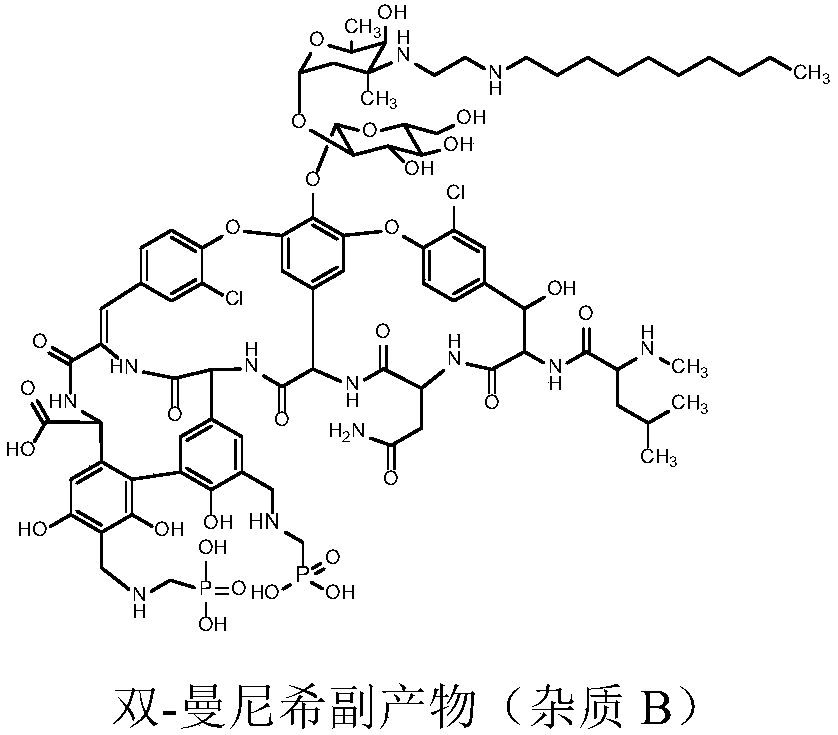
[0056] Add 22L of N,N-dimethylformamide and 6.16kg of the compound of formula I into a 50L reaction tank, stir for 10 minutes, cool down to 10-20°C, slowly add 1.13L of diethylamine dropwise to the feed solution, and dropwise, Raise the temperature, control the temperature of the feed solution to 23-33°C, stir and react for 2 hours, take samples, and track and detect by HPLC until the end of the reaction. Filter and collect the filtrate.
[0057] Add 327L of isopropyl ether to a 500L reactor, control the temperature at 10-20°C, slowly add the collected filtrate dropwise to the isopropyl ether while stirring, and keep the temperature of the material liquid at 10-20°C to stir and crystallize for 2 hours Above, after the crystallization is completed, shake off the filter, and the filter cake is beaten twice with 65L isopropyl ether on average, and dried under reduced pressure at 25±5°C for more than 12 hours to ob...
Embodiment 3
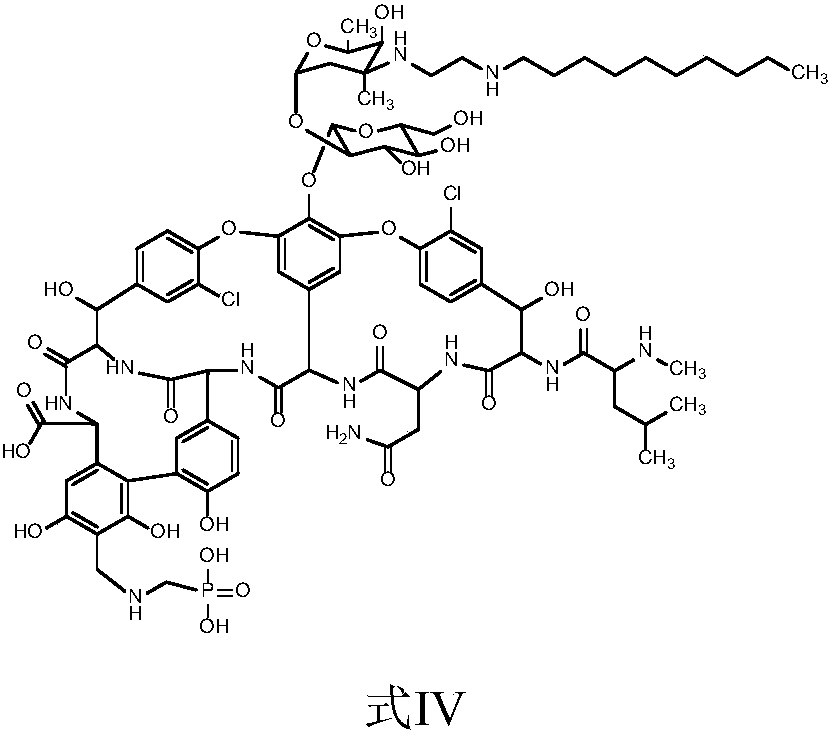
[0058] The preparation of embodiment 3 compound IV
[0059]
[0060] Preparation of aminomethylphosphonic acid solution: at room temperature, add 13L of purified water into a 20L glass reaction tank, add 1.5kg of aminomethylphosphonic acid under stirring, stir for 10 minutes, slowly add N,N-diisopropylethyl Amine 2.17L, stirred until the material solution is clear, cooled to 0-5°C, set aside.
[0061] In a 200L reaction tank, add 35.2L of acetonitrile, 30.4L of purified water, and 5.02kg of the compound of formula III, stir for 10 minutes, cool down, control the temperature of the feed liquid at -15~-5°C, and slowly add 4.48L of N , 4.48L of N-diisopropylethylamine, after dropping, stir until the material solution is clear. Control the feed liquid temperature at -15 to -5°C, add the above aminomethylphosphonic acid solution dropwise to the solution of the compound of formula III, control the feed liquid temperature at -15 to -5°C and stir for 10 minutes. Control the tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com