Active substance of lithium ion battery anode and preparation method thereof
A cathode active material, lithium-ion battery technology, applied in battery electrodes, electrode manufacturing, circuits, etc., can solve the problems of thermal stability, rate discharge performance cycle performance, poor overcharge performance, etc., to achieve excellent performance, excellent overcharge performance and the effect of high temperature performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
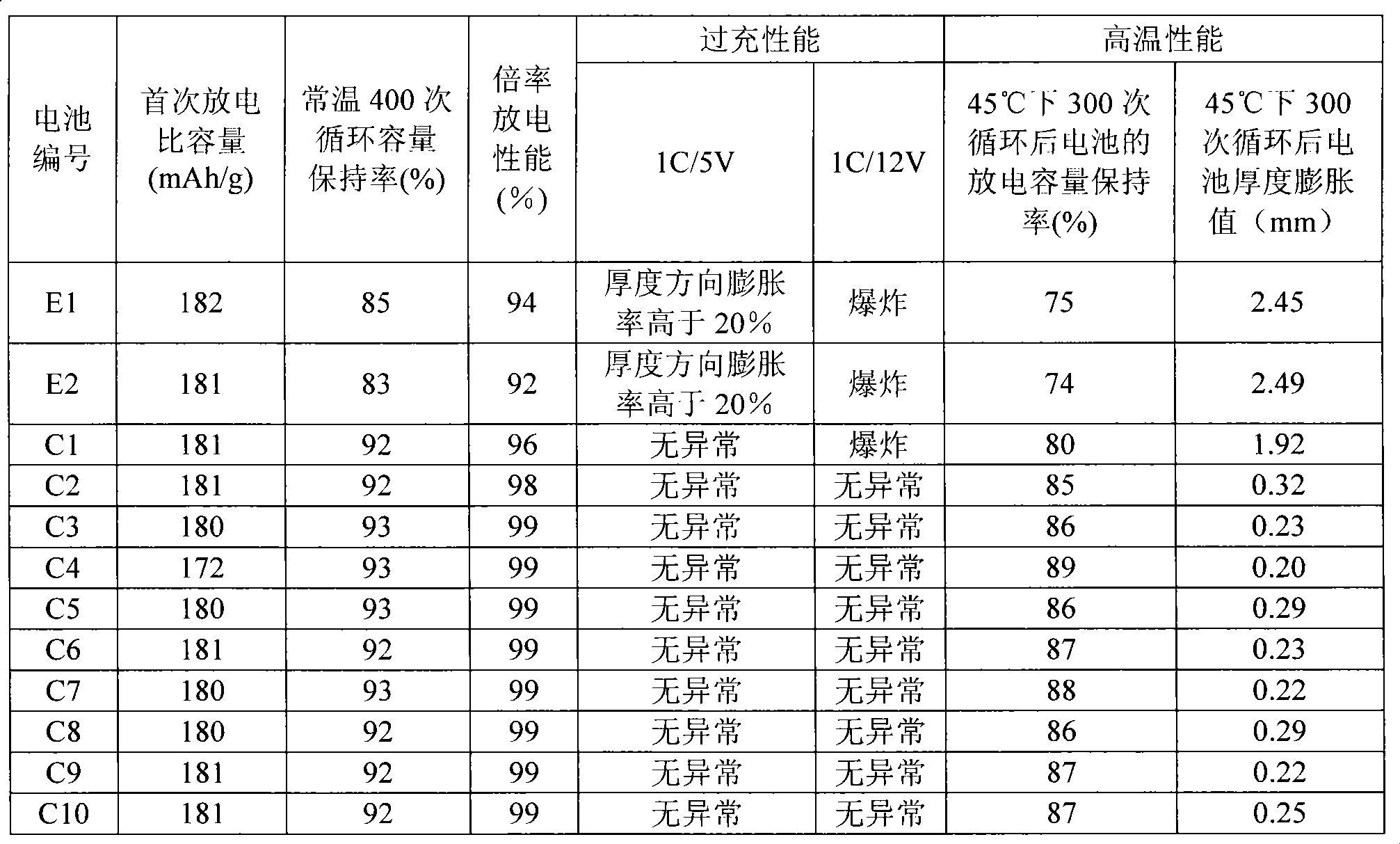
Examples
Embodiment 1
[0078] This example illustrates the preparation method of the positive electrode active material of the present invention.
[0079] Dissolve lithium nitrate, nickel nitrate, manganese nitrate, cobalt nitrate and magnesium nitrate into deionized water according to the molar ratio Li:Ni:Mn:Co:Mg=1.02:0.3:0.3:0.3:0.1, and add nickel ions, manganese ions, citric acid twice the total number of moles of cobalt ions as a complexing agent. Then the oxide D1 prepared in Comparative Example 1 was added under stirring at 80°C, and the stirring was continued for 120 minutes to obtain a blue-black gel. The molar ratio of the oxide D1 to lithium nitrate is 100:1.02. The gel was dried at 120° C., and then sintered at 800° C. for 10 hours in an oxygen atmosphere to obtain a positive electrode active material M1. According to XRD diffraction analysis and ICP atomic emission spectrometry analysis, the positive electrode active material M1 contains the general formula LiNi 0.80 co 0.15 al 0...
Embodiment 2
[0082] Prepare the positive electrode active material according to the method of Example 1, the difference is that by adjusting the molar ratio of the oxide D1 and lithium nitrate so that in the obtained positive electrode active material M2, the general formula is LiNi 0.80 co 0.15 al 0.05 o 2 The oxide IA has the general formula Li(NiMn) 0.3 co 0.3 Mg 0.1 o 2 The molar ratio of oxide IB is 100:5. The average particle diameter of the oxide IA was measured to be 14 um, and the average particle diameter of the oxide IB was 100 nm.
Embodiment 3
[0084] Prepare the positive electrode active material according to the method of Example 1, the difference is that by adjusting the molar ratio of the oxide D1 and lithium nitrate so that in the obtained positive electrode active material M3, the general formula is LiNi 0.80 co 0.15 al0.05 o 2 The oxide IA has the general formula Li(NiMn) 0.3 co 0.3 Mg 0.1 o 2 The molar ratio of oxide IB is 100:10. The average particle diameter of oxide IA was measured to be 14 um, and the average particle diameter of oxide IB was 200 nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com