Preparation method of catalyst for hydrogen production from methanol-steam reforming
A technology for reforming hydrogen production and catalysts, which is applied in catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., and can solve problems such as price restrictions, general promotion and application, easy deactivation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
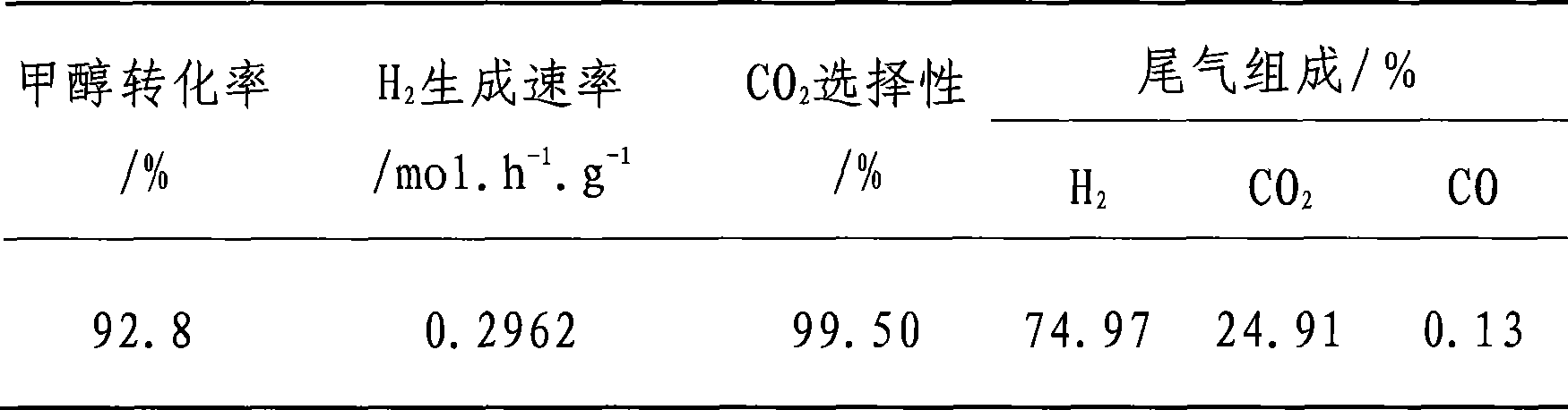
Embodiment 1
[0012] a. The catalyst is prepared by co-current co-precipitation method, and copper nitrate, zinc nitrate and aluminum nitrate are dissolved in distilled water according to the molar percentage of metal atoms Cu:Zn:Al=45.0:45.0:10.0, at 59~61°C with 1mol.L -1 The sodium carbonate solution was added dropwise for co-precipitation, fully stirred during the precipitation process, and the pH value was kept at about 7.0, after 1 hour of reaction, suction filtration, the filter cake was washed with deionized water for 6 to 7 times, and dried at 120°C 12h, and finally baked at 350°C for 4h to prepare Cu / ZnO / Al 2 o 3 catalyst;
[0013] b. add the Cu / ZnO prepared by step a in the mixed solution of zirconium oxychloride and cerium nitrate according to the molar percentage of metal atoms Cu:Zn:Al:Zr:Ce=40.9:40.9:9.2:7.2:1.8 / Al 2 o 3 Catalyst (particle size>200 mesh) and keep stirring to form a suspension, then slowly drop 1mol.L at 60°C -1 Sodium carbonate solution, until the pH v...
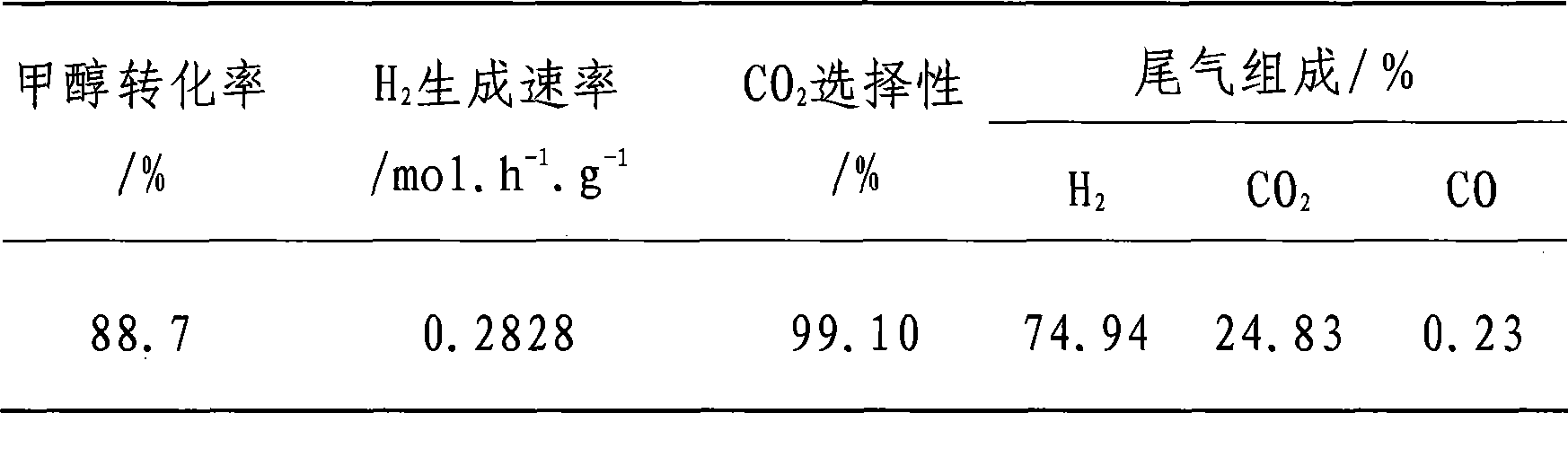
Embodiment 2
[0020] The catalyst preparation method and activity evaluation method are the same as in Example 1, and the molar percentage ratio of metal atoms is Cu:Zn:Al:Zr:Ce=37.7:37.7:19.0:4.7:0.9. Reaction condition: H 2 O / CH 3 OH=1.1 / 1 (molar ratio), WHSV=3.60h -1 .g -1 , T=230°C, P=0.1MPa, see Table 2 for the evaluation results.
[0021] Table 2
[0022]
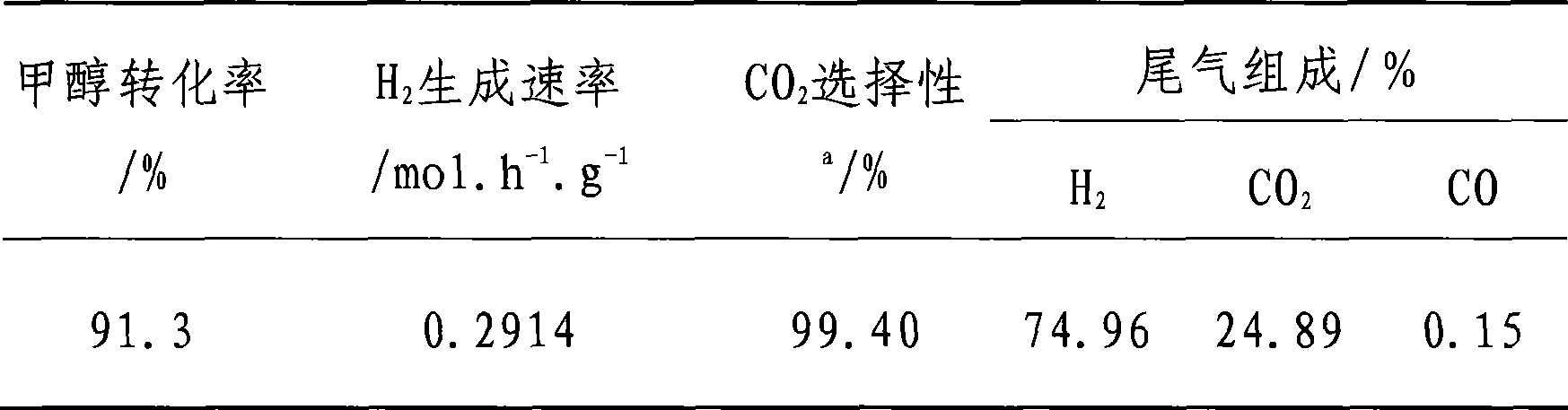
Embodiment 3
[0024] The catalyst preparation method and activity evaluation method are the same as in Example 1, and the molar percentage ratio of metal atoms is Cu:Zn:Al:Zr:Ce=43.2:43.2:4.5:7.3:1.8. Reaction condition: H 2 O / CH 3 OH=1.1 / 1 (molar ratio), WHSV=3.60h -1 .g -1 , T=230°C, P=0.1MPa, see Table 3 for the evaluation results.
[0025] table 3
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com