Mixed yolk antibody for preventing and treating duck infectious serositis and preparation thereof
A yolk antibody and infectivity technology, which is applied in the field of mixed yolk antibody for prevention and treatment of infectious serositis in ducks and its preparation, can solve the problem that the research on yolk antibody of R. anatipestifer has not yet been reported, and can reduce the use of antibiotics , high protection rate, and the effect of promoting development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of Riemerella anatipestifer egg yolk antibody
[0031] (1) Pure culture, concentration and inactivation of R. anatipestifer bacterial antigens;
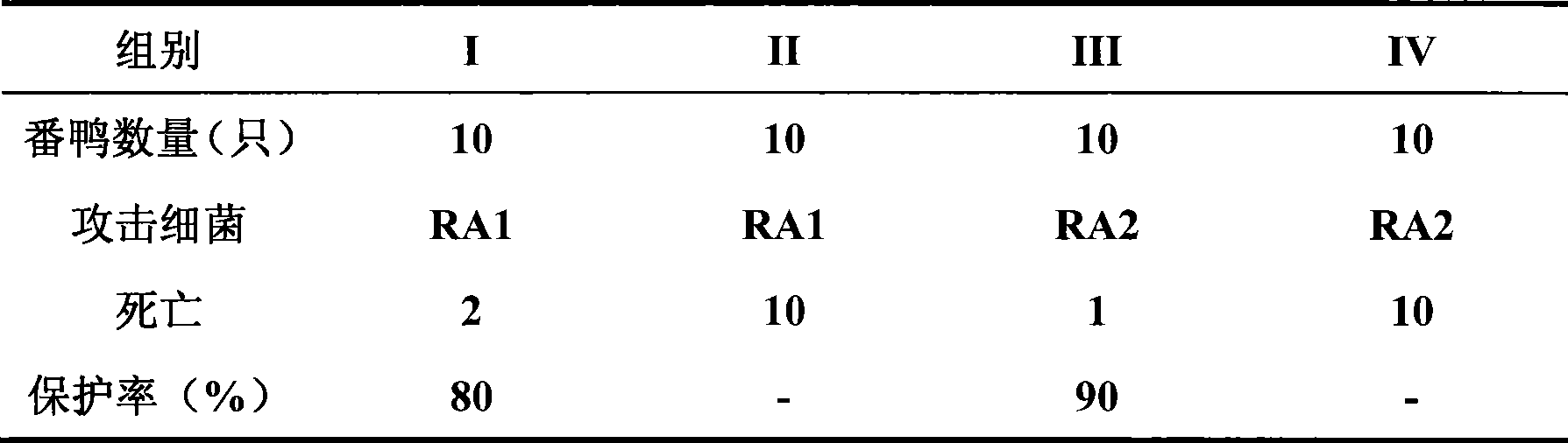
[0032] Riemerella anatipestifer RA1 (serotype I) and RA2 (serotype II) strains were respectively inoculated into tryptone yeast broth containing 0.5% serum, and cultured at 36~37℃ with shaking for 18 hours to logarithmic growth phase Centrifuge at 3000 rpm for 15 minutes at 4°C, dissolve the precipitate in PBS buffer, then centrifuge for 15 minutes under the same conditions, collect the bacteria, and slowly add the analytical pure formaldehyde solution to 0.3% of the total bacterial solution, at 36~37 Inactivate for 36 hours at a constant temperature of ℃;
[0033] (2) Mix the antigen in proportion and add the immune adjuvant;
[0034] Mix the inactivated RA1 and RA2 bacterial antigens in a ratio of 1:2, add the sterilized complete Freund's adjuvant to some equal parts, and add the incomplete Freund's adjuvan...
Embodiment 2
[0040] Example 2 Preparation of Riemerella anatipestifer egg yolk antibody
[0041] (1) Pure culture, concentration and inactivation of R. anatipestifer bacterial antigens;
[0042] Take Riemerella anatipestifer (serotype I) and RA2 (serotype II) strains, respectively, inoculate them in tryptone yeast broth containing 0.5% serum, and culture at 36~37℃ for 18 hours to the logarithmic growth phase. Centrifuge at 3000 rpm for 15 minutes at 4°C, dissolve the precipitate in PBS buffer, then centrifuge for 15 minutes under the same conditions, collect the bacteria, and slowly add the analytical pure formaldehyde solution to 0.3% of the total bacterial solution. Inactivate for 36 hours at a constant temperature of ~37℃;
[0043] (2) Mix the antigens in proportion and add immune adjuvants;
[0044] After inactivation, mix RA1 and RA2 bacterial antigens in a ratio of 1:4, add sterilized propolis in equal volume, and emulsify to obtain bacterial antigens;
[0045](3) Choose healthy non-immu...
Embodiment 3
[0050] Example 3 Preparation of Riemerella anatipestifer egg yolk antibody
[0051] (1) Pure culture, concentration and inactivation of R. anatipestifer bacterial antigens;
[0052] Take Riemerella anatipestifer (serotype I) and RA2 (serotype II) strains, respectively inoculate them in tryptone yeast broth containing 0.5% serum, and culture at 36~37℃ for 18 hours to logarithmic growth phase with shaking Centrifuge at 3000 rpm for 15 minutes at 4°C, dissolve the precipitate in PBS buffer, then centrifuge for 15 minutes under the same conditions, collect the bacteria, and slowly add the analytical pure formaldehyde solution to 0.3% of the total bacterial solution, at 36~37 Inactivate for 36 hours at a constant temperature of ℃;
[0053] (2) Mix the antigen in proportion and add the immune adjuvant;
[0054] After inactivation, mix the RA1 and RA2 bacterial antigens in a ratio of 1:6, add the sterilized white oil for injection in equal volume, and emulsify the bacterial antigens;
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com