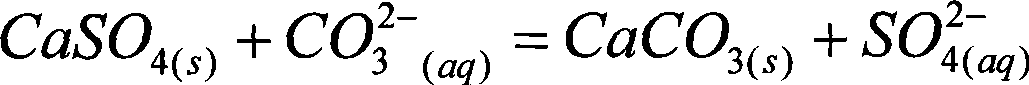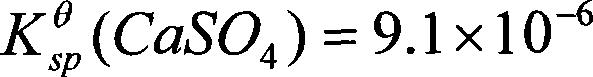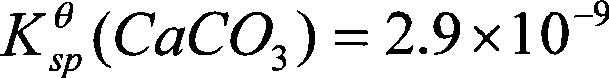Method for coproduction of ammonia sulfate and superfine light calcium carbonate from fluorgypsum
A light calcium carbonate and fluorogypsum technology, applied in the direction of calcium carbonate/strontium/barium, ammonium sulfate, etc., can solve the problems of fluorogypsum insoluble in water, polluted soil and groundwater environment, slow hydration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The method that utilizes fluorogypsum of the present embodiment to produce superfine light calcium carbonate to co-produce ammonium sulfate may further comprise the steps:
[0031] ①Crush the fluorine gypsum into fluorine gypsum powder passing through a -40 mesh sieve;
[0032] ② Wash and filter the fluorogypsum powder until the pH value of the filtrate is 4, then add an appropriate amount of water to the filter cake to make a thin paste with a calcium sulfate solid content of 10%;
[0033] ③ Add 0.2% sodium lauryl sulfate to the thin paste, add a mixture of ammonium carbonate and ammonium bicarbonate whose total amount is 1.05 times the amount of calcium sulfate, and its mass ratio is 10:1, Stir the reaction at 40°C for 3 hours, and the gas generated is used to recover ammonium carbonate or ammonium bicarbonate;
[0034] ④ After the reaction is completed, filter, wash the precipitate until the washing water is detected with a barium chloride solution without white pre...
Embodiment 2
[0037] The method that utilizes fluorogypsum of the present embodiment to produce superfine light calcium carbonate to co-produce ammonium sulfate may further comprise the steps:
[0038] ①Crush the fluorogypsum into fluorogypsum powder passing through a -60 mesh sieve;
[0039] ② Wash and filter the fluorogypsum powder until the pH value of the filtrate is 4.5, then add an appropriate amount of water to the filter cake to make a thin paste with a calcium sulfate solid content of 15%;
[0040] 3. add the ammoniacal liquor of 0.1 times of the amount of calcium sulfate substance, 1% sodium lauryl sulfate in thin paste, the total amount is the ammonium carbonate and ammonium bicarbonate mixture of 1.1 times of the amount of calcium sulfate substance, and its mass ratio is 5:1, stirring and reacting at 45°C for 4 hours, the gas generated is used to recover ammonium carbonate or ammonium bicarbonate;
[0041] ④ After the reaction is completed, filter, wash the precipitate until th...
Embodiment 3
[0044] The method that utilizes fluorogypsum of the present embodiment to produce superfine light calcium carbonate to co-produce ammonium sulfate may further comprise the steps:
[0045] ① Grinding the fluorine gypsum into fluorine gypsum powder passing through a -90 mesh sieve;
[0046] ② Washing and filtering the fluorogypsum powder until the pH value of the filtrate is 5, then adding an appropriate amount of water to the filter cake to make a thin paste with a calcium sulfate solid content of 20%;
[0047] 3. add the ammoniacal liquor of 0.4 times of the amount of calcium sulfate substance, 2% sodium lauryl sulfate in thin paste, the total amount is the ammonium carbonate and ammonium bicarbonate mixture of 1.15 times of the amount of calcium sulfate substance, and its mass ratio is 3:1, stirring and reacting at 50°C for 5 hours, the gas generated is used to recover ammonium carbonate or ammonium bicarbonate;
[0048] ④ After the reaction is completed, filter, wash the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com