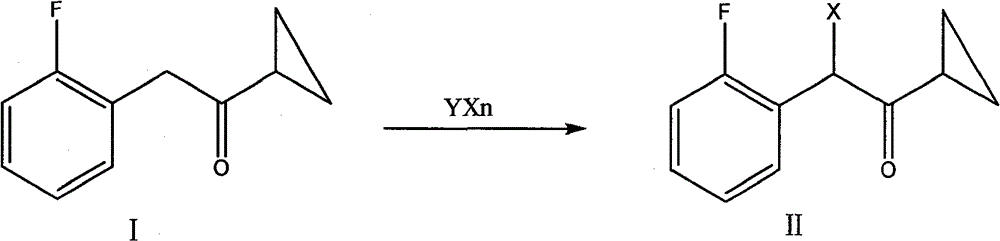
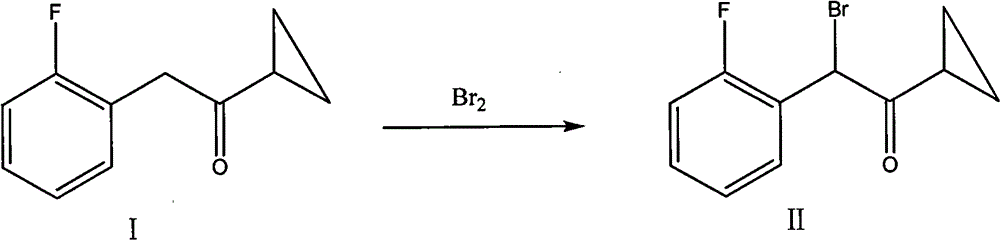
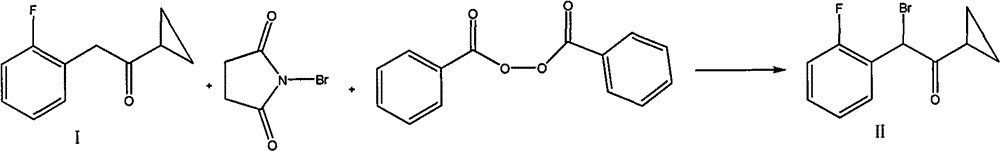
Preparation of alpha-cyclopropyl carbonyl-2-benzyl-fluorobenzyl halogen
A technology of fluorobenzyl halogen and cyclopropylcarbonyl, which is applied in the field of preparation of α-cyclopropylcarbonyl-2-fluorobenzyl halogen, can solve the problems of complicated post-processing and relatively high price of N-bromosuccinimide. Expensive, unfavorable for industrial production and other problems, to achieve outstanding yield, suitable for large-scale industrial production, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of cyclopropylcarbonyl-2-fluorobenzyl bromide
[0022] 17.8 g of cyclopropyl-2-fluorobenzyl ketone was dissolved in 100 ml of chloroform and heated to reflux, and an ethyl acetate solution of 44.7 g of copper bromide was added dropwise. After detecting the reaction until the raw materials disappeared, the cuprous bromide was filtered while it was hot and washed with chloroform, the filtrates were combined, and the solvent was evaporated to dryness to obtain 23.9 g of the target compound with a yield of 93% (HPLC showed a purity of 95.2%).
[0023] NMR (CDCl3) δppm:
[0024] 0.82-1.25 (4H, multiplet);
[0025] 2.12-2.18 (1H, multiplet);
[0026] 5.94 (1H, singlet);
[0027] 7.07-7.52 (4H, multiplet);
Embodiment 2
[0028] Embodiment 2: the preparation of cyclopropylcarbonyl-2-fluorobenzyl bromide
[0029] 17.8 g of cyclopropyl-2-fluorobenzyl ketone was dissolved in 100 ml of ethanol and heated to 60° C., and an ethanol solution of 44.7 g of copper bromide was added dropwise. Check the reaction until the raw materials disappear, filter the cuprous bromide while it is hot, and wash with ethanol, combine the filtrates, evaporate the solvent to dryness to obtain the target compound. (yield 98.5%, HPLC shows that the purity is 98.0%)
Embodiment 3
[0030] Embodiment 3: the preparation of cyclopropylcarbonyl-2-fluorobenzyl bromide
[0031] The operation is the same as in Example 2, except that tetrahydrofuran is used instead of ethanol, other conditions are the same as in Example 2, and the solvent is evaporated to dryness to obtain the product. (88.1% yield, 94.3% purity by HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com